INVEGA SUSTENNA- paliperidone palmitate injection Janssen Pharmaceuticals, Inc. Warning: increased mortality in elderly Parenteral drug products should be inspected visually for foreign matter and discoloration prior to administration, whenever product and container permit.
How to use Invega Sustenna Syringe. Read the Patient Information Leaflet provided by your pharmacist before you start using paliperidone and each time you Does Invega Sustenna Syringe interact with other drugs you are taking? Enter your medication into the WebMD interaction checker.
Like Invega Sustenna, it is administered by a healthcare professional in the upper arm or buttocks. It may be administered once patients have been adequately treated with Invega Sustenna a for at least four months. Invega Trinza means patients don't have to attend as many appointments and they don'
Invega Sustenna is an injectable form of the drug that is administered every 4 weeks and is thought to be advantageous it mitigates risk of users forgetting to take a daily pill. The drug functions primarily as a D2 dopamine receptor and 5-HT2A serotonin receptor antagonist.
Medscape - Schizophrenia dosing for Invega, Invega Sustenna (paliperidone), frequency-based adverse effects, comprehensive interactions Dose initiation dependent on once-monthly Invega Sustenna dose. Initiate Invega Trinza when the next 1-month paliperidone dose is scheduled with
How is it given. Invega Sustenna will be given to you by injection by a healthcare professional. A third Invega Sustenna injection of 75 mg in either the deltoid or gluteal muscles should be administered 5 weeks after the first injection (regardless of the timing of the second injection).
INVEGA SUSTENNA®. Modified Release Aqueous Suspension for Intramuscular Injection. Paliperidone palmitate. Consumer Medicine Information (CMI). What is in this leaflet. This leaflet answers some of the common questions about Invega Sustenna. It does not contain all of
Invega Sustenna® é contraindicado em pacientes com hipersensibilidade conhecida à paliperidona ou a qualquer dos componentes da formulação. O Invega Sustenna® não deve ser misturado a nenhum outro produto ou diluente e destina-se à administração intramuscular diretamente da
Source: Invegasustenna Consumer - Content - How INVEGA SUSTENNA® Works. Invega Sustenna Side Effects: Common, Severe, Long Term - Invega Sustenna is k/a Paliperidone palmitate which is an antipsychotic medication used in Schizophrenia. It's onset of action
Invega Sustenna 100Mg Injection is an atypical antipsychotic and a dopamine antagonist. It is used to treat mental and mood disorders such as schizophrenia The dosage for Invega Sustenna 100Mg Injection should be prescribed by your doctor based on your specifications. Usually the adult dose
Invega Sustenna injection is an atypical anti-psychotic Rx medication. Get information and discount Invega Sustenna prices online at Invega Sustenna is not approved as treatment for the mental problems associated with dementia. Until you know how your body will react to Invega Sustenna
INVEGA SUSTENNA® is intended for intramuscular use only. Do not administer by any other route. Avoid inadvertent injection into a blood vessel. Administer the dose in a single injection; do not administer the dose in divided injections. Inject slowly, deep into the deltoid or gluteal muscle.
Invega Sustenna how to video shot for Janssen, Johnson & Johnson. Multi-camera studio shoot.
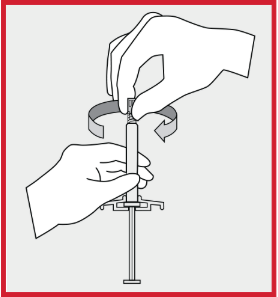
invega sustenna administer
INVEGA SUSTENNA (paliperidone palmitate injection) comes in different strengths and amounts. The appearance of INVEGA SUSTENNA can differ based on the dosing. Your doctor may change the dosage and prescription of INVEGA SUSTENNA to get you the best results possible.
Educational Dose Illustrator for: INVEGA SUSTENNA® (paliperidone palmitate) INVEGA TRINZA® The Educational Dose Illustrator can be used to visualize how dosing affects paliperidone plasma concentrations Gastrointestinal: INVEGA® should ordinarily not be administered to patients
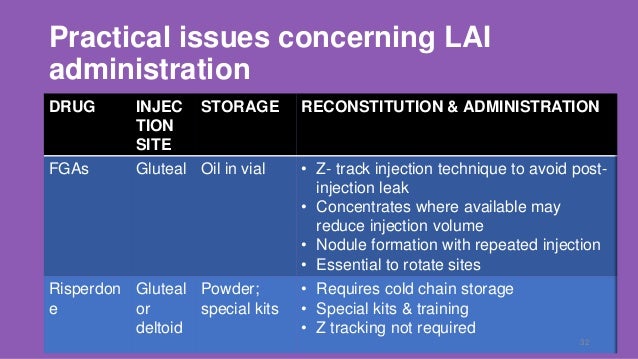
antipsychotics paralytic
Invega Sustenna ("Sustenna" or paliperidone palmitate) is a long-acting injectable (LAI) formulation of paliperidone. It was the first available second-generation antipsychotic LAI to provides coverage for 4 weeks. It is FDA-indicated to treat adults with diagnoses of schizophrenia and schizoaffective disorder.
invega sustenna paliperidone palmitate dosage aerate plunger rod

invega sustenna bula paliperidone palmitate 150mg injetavel enchida controlado seringa injectable janssen xeplion felleskatalogen posologia pakningsvedlegg rxlist

invega syringe shake vigorously paliperidone seconds least palmitate effects side rxlist

procrit remicade epoetin alfa janssen carepath simponi aria infliximab

paliperidone palmitate injections mental
Medical information for Invega Sustenna including its dosage, uses, side, effects, interactions, pictures and warnings. It is recommended to administer a third injection of 117 mg in either the deltoid or gluteal muscle 5 weeks after the first injection (regardless of the timing of the second injection).
See the step-by-step instructions for administering INVEGA SUSTENNA® (paliperidone palmitate) to patients. See full prescribing and safety INVEGA SUSTENNA® is intended for intramuscular use only. Do not administer by any other route. Avoid inadvertent injection into a blood vessel.
INVEGA SUSTENNA® (paliperidone palmitate) Patient Support Supporting Treatment Helping you help your patients get started with the INVEGA SUSTENNA® treatment you prescribed We understand how important it is for your patients to take their INVEGA SUSTENNA® treatment as you've prescribed.
INVEGA SUSTENNA prescription and dosage sizes information for physicians and healthcare professionals. Psychosis: Indications for: INVEGA SUSTENNA. Schizophrenia. Schizoaffective disorder as monotherapy or as an adjunct to mood stabilizers or antidepressants.
See full prescribing information for INVEGA SUSTENNA®. INVEGA SUSTENNA® (paliperidone palmitate) extended-release injectable suspension, for intramuscular use INVEGA SUSTENNA® must be administered using only the needles that are provided in the INVEGA SUSTENNA® kit.
Brand Name: Invega Sustenna. Drug Class: How Do Second Generation Antipsychotics Work? The recommended dosing of INVEGA SUSTENNA® for each approved indication is displayed in Table 1. The recommended initiation of INVEGA SUSTENNA® is with a dose of 234 mg on treatment day
How Invega (paliperidone) injection works. Invega (paliperidone) injection is only available as an injection in the muscle that is administered by a healthcare provider. If you have been on Invega Sustenna for at least 4 months, ask your healthcare provider if you can use Invega Trinza (
INVEGA SUSTENNA has been shown to be effective in delaying time to recurrence of symptoms of schizophrenia in long-term use. A third INVEGA SUSTENNA injection of 75 mg in either the deltoid or gluteal muscles should be administered 5 weeks after the first injection (regardless of the timing

injection paliperidone palmitate extended release needle secure invega surface complete flat

ashleenichols ashlee nichols bedroomdecortips shisu
What Invega Sustenna is used for. Invega Sustenna belongs to a group of medicines called How is it given. Using it for the first time. Treatment with Invega Sustenna will not be started by your doctor How many injections you will need. Invega Sustenna helps control your condition but will not cure it.
occur when co-administered with INVEGA SUSTENNA®. () x Strong CYP3A4/P-glycoprotein (P-gp) inducers: Avoid using a strong. Schizophrenia Schizoaffective Disorder 16 HOW SUPPLIED/STORAGE AND HANDLING 17 PATIENT COUNSELING INFORMATION.
Invega Sustenna Administration/Patient Management. Does anyone have any experience administering and/or managing a patient on Invega Sustenna? I'm the only pharmacist trained to administer it, currently, and I'm sort of nervous because there's a lot more to know than what
A summary of dosage and administration information for INVEGA SUSTENNA® (paliperidone palmitate 1-month). INVEGA SUSTENNA® (paliperidone palmitate). Search Medical Information.
Invega, Invega Sustenna, INVEGA TRINZA. HOW SUPPLIED. Invega Sustenna/INVEGA TRINZA Intramuscular Inj Susp: , , , If the last dose of Invega Trinza was 819 mg: Administer Invega Sustenna 156 mg on Day 1 into the deltoid muscle and administer
INVEGA SUSTENNA® must be administered using only the needles that are provided in the INVEGA SUSTENNA® kit. INVEGA SUSTENNA® is available as a white to off-white aqueous extended-release injectable suspension for intramuscular injection in dose strengths of 39 mL,

