So testing should be tested for chloride ion and sulfate ion to identify two anions. Add barium hydroxide to samples of HCl and H2SO4. Lithium sulfate is soluble in water and it completely decomposes to lithium and sulfate ion. How do you identify copper sulfate?

copper zinc sulfate reaction between
in Water Analysis We provide industrial firms all over the world with water analysis test kits that are accurate and easy to use. Our customers can depend on us for superior quality not only in our products but in every aspect of their relationships with us including friendly personal service, prompt deliveries and well-informed ...
Watch this how-to-video to learn how to test for sulfate in FRESH water using the eXact iDip® Smart Photometer System®. The eXact iDip® Smart
How to identify water in a substance - Anhydrous copper sulphate - Copper sulphate formula - Hydrated copper sulphate - Chemical test for water with anhydrous copper sulphate - Some results The test for water requires a chemical compound: Copper sulfate anhydrous. This is a white powder.
Aluminum sulfate (alum) is also used as a coagulant in water treatment plants. The EPA lists aluminum as a secondary contaminant, meaning it has few Conduct a water test to find out if cadmium is present in your tap water. A solution for reducing the presence of this toxic contaminant is a
How do you test for sulphate ions in water? TEST OF SULPHATE IONS IN WATER:- Sulphates of Barium (BaSO4), Strontium (SrSO4), and Lead (PdSO4) are insoluble in water, those of Calcium (CaSO4) and Mercury (HgSO4) are partially soluble in water.
Testing for Sulfate and Hydrogen Sulfide. Interpreting Test Results. Sulfate minerals can cause scale buildup in water pipes similar to other minerals and may be associated with a bitter However, not all laboratories are approved to test for sulfate. Select a laboratory and contact them to obtain
Water hardness test kits may use water hardness test tablets or paper test strips (see below). When mixed with (a test tablet) or wet by (a test strip such as those shown at left) water of a specified volume (fill the Sears offers free water testing to its customers, testing for hardness, acidity, and clarity.

ammonium nh4 2so4 sulfate equation
Our supplier suggests a copper sulfate test after passivation vs a water emergence test to save time. We have always specified water emergence testing after passivation and I wonder if I could accept The reasoning is there is a risk of false failures. I use a hot water immersion test for such parts.
Sulfates. Introduction. How does sulfate enter our water supplies? Health risks for humans who drink water containing high sulfate levels. Sulfate is one of the major dissolved components of rain. High concentrations of sulfate in the water we drink can have a laxative effect when combined
How much sulfate is safe in drinking water? A health-based advisory for acute effects (absence of laxative effects) of 500 mg of sulfate/L is recommended. Sulfate in drinking water currently has a secondary maximum contaminant level (SMCL) of 250 milligrams per liter (mg/L), based on
Sulfates are discharged into water in industrial wastes and through atmospheric deposition. Sulfate concentration in seawater is about 2,700 milligrams per liter. Long-term and short-term exposure studies to determine a hazard assessment for sulfate are currently available in humans and animals.
• Sulfate minerals can cause scale buildup in water pipes. • Sulfate can make cleaning clothes difficult. Using chlorine bleach in sulfur water may reduce the It is very difficult to test for hydrogen sulfide in a laboratory because the hy-drogen sulfide escapes very quickly from water and may be gone by
Learn about Coagulation Process Operation and Jar Testing in this excerpt from our Water Treatment Exam Review course. Watch this how-to-video to learn how to test for sulfate in FRESH water using the eXact iDip® Smart Photometer System®.
This test method was developed for concentrations of water-soluble sulfate in soils between and % sulfate by mass. Cement D 1193 Specication for Reagent Water E 11 Specication for Wire and Cloth Sieves for Testing. Purposes E 60 Practice for Analysis of Metals, Ores, and Related.
Method of test for determination of sulfate ion in soils and water 1. Scope The analytical procedure for water samples and for the extracts from soil samples is
How Does Sulfate Become a problem? Sulfate minerals can cause scale buildup in water pipes similar to other minerals and may be associated with a Notes on Level 1 Testing for Sulfate, Hydrogen Sulfide, Sulfate Reducing Bacteria, and Sulfur. You will suspect a problem with sulfate if the
However, most of the test methods are based on determining water soluble sulfates in the soil. The extraction techniques are often derived from water 23 to water as possible in an attempt to solubilize all available sulfate ions is recommended. Although the different test methods follow

hydroxide cuso4 sodium sulphate reaction naoh cupric
How to test water hardness & how to measure it using test strips, a water hardness soap test solution and other methods. This article explains how to measure This means if you're faced with wanting to balance water in a spa by increasing the calcium hardness from 60 ppm to 150 ppm, the strip can'
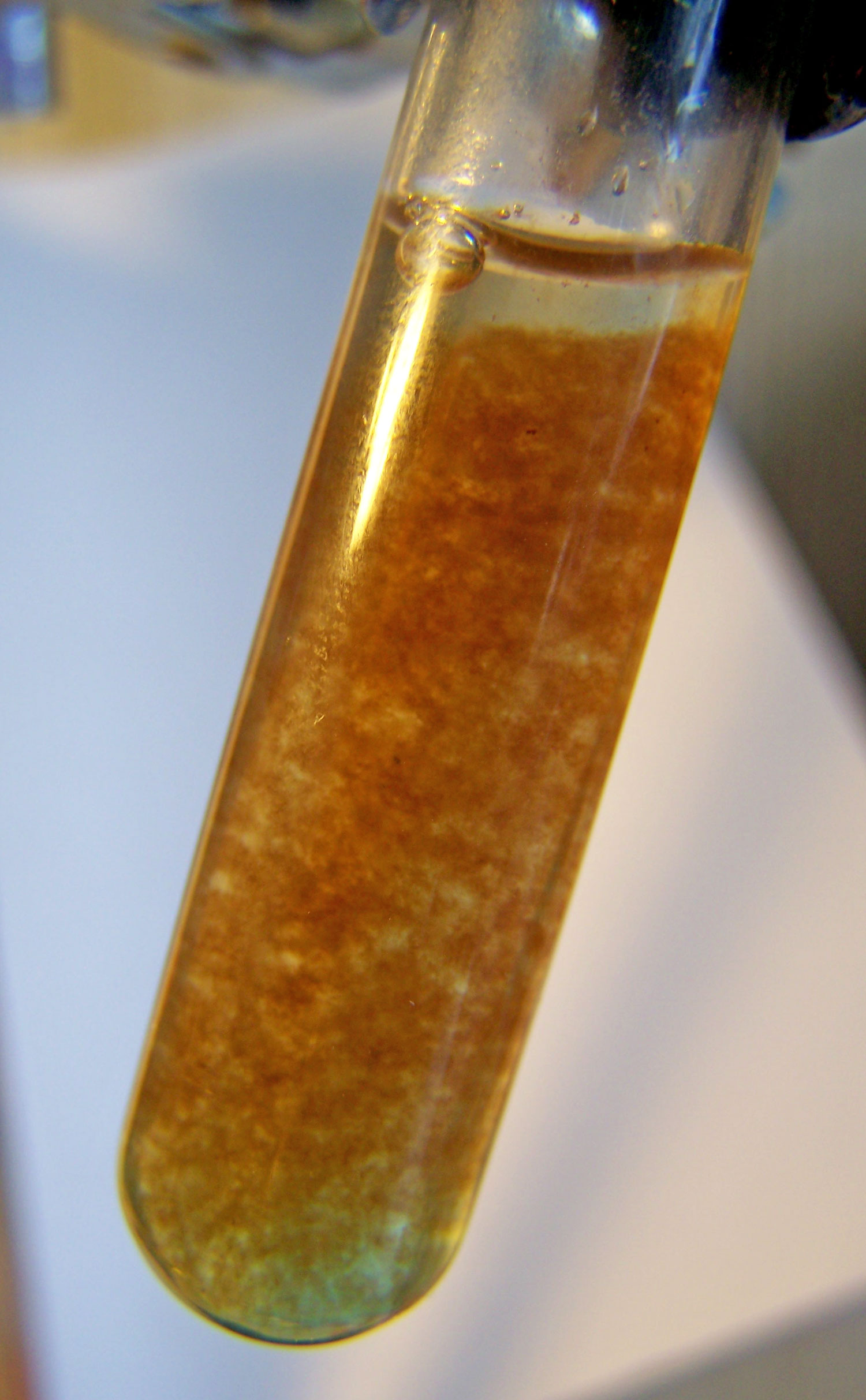
sulfate precipitate copper solution reaction brown hydroxide iron ferric chemical state oxidized oral ciencia postulated identity forms sample bibliotecapleyades
How to detect if you have too much sulfates/sulfides. Private well owners are responsible for the quality of their drinking water. Smell and bad taste can also be signs of wastewater pollution which is why well owners should also consider testing for bacterial contaminations.
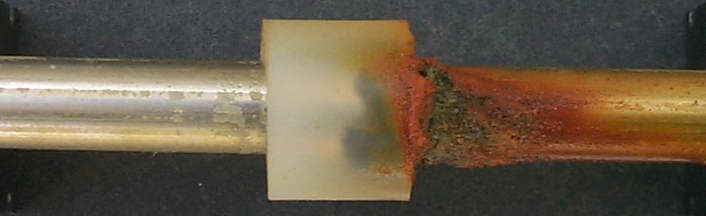
corrosion stainless steel test crevice testing methods duplex interpretation results lean result figure
Prepare test solution - Dissolve 8 grams of copper sulfate in 500 ml of distilled water in which 2 - 3 ml of sulfuric acid has been added. Establish a baseline by performing the test on raw stock, passivated steel, and steel that obviously has free iron present. Document how the copper sulfate
Sulfate in Drinking-water, Background document for development of WHO Guidelines for Drinking-water Quality, is an update of the background document published in the second edition of the Guidelines. 5. effects on laboratory animals and in vitro test systems.
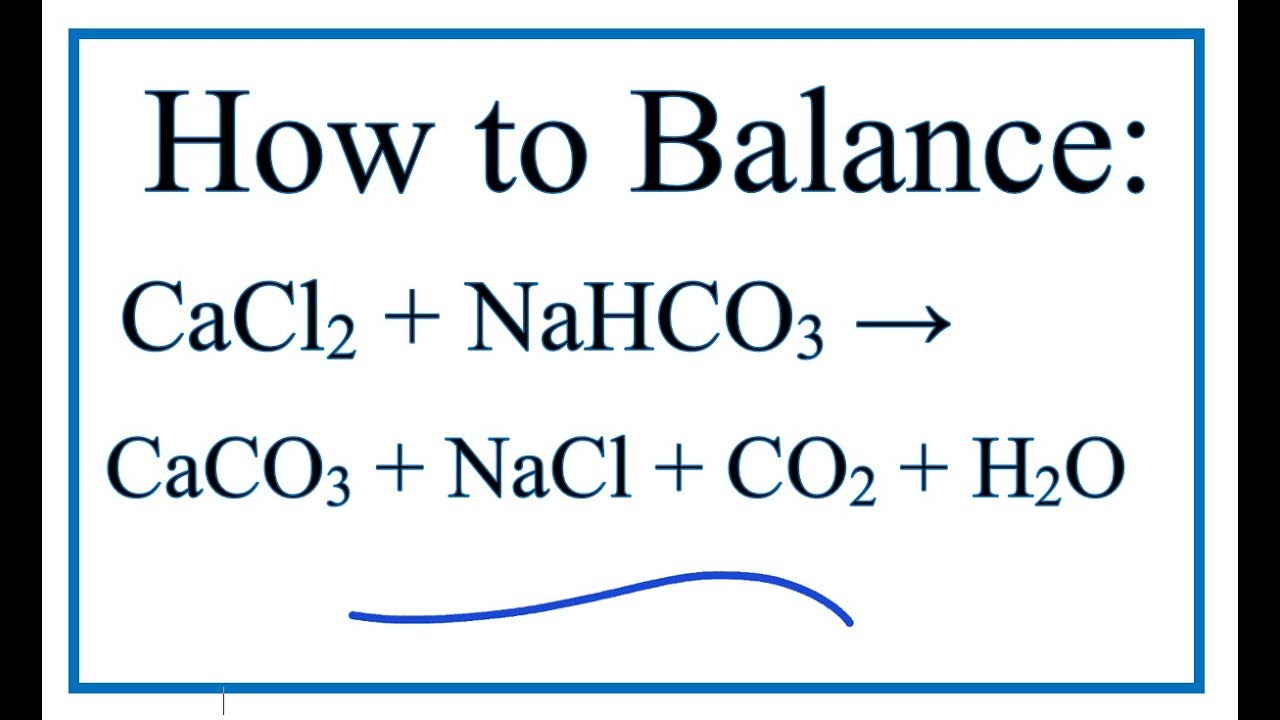
cacl2 nahco3 nacl caco3 co2
This mail-in water testing kit will screen your sample for over 200 contaminants and provide in-depth results. The kit tests for 6 in-organic substances, 12 physical properties, 32 toxic metals , 20 semi-volatile It also includes an at-home test for coliform (bacteria) and instructions on how to complete it.
For sulfate, the scientific evidence was insufficient to set an AI. However, sulfate needs are met by the current recom-mended intakes for sulfur amino acids, which Although a low intake of total water has been associated with some chronic diseases, this evidence is insufficient to establish water
Sulfate In Well Water Well Management Program. Sulfate occurs naturally in most of Minnesota's groundwater. You can find out the level of sulfate in your water by having the water tested at a laboratory. Health Risks for Humans. People who are not used to drinking water with high sulfate
A sample containing an alkali sulfate is dried, weighed and dissolved in dilute HCl. Barium chloride solution is added in excess to precipitate barium sulfate, and the precipitate is digested in the hot solution. The precipitate is filtered through a paper filter which is then ignited and completely ashed.
The SMCL for sulfate is 250 mg/L because that concentration can impart a bitter-to-salty taste to the water; at a concentration Table 3. Results of the spike recovery experiments for chloride and sulfate in water and soil samples using the Dionex IonPac AS22 column with electrolytically

moisture dryer braids fulani scalp locs pulling
Chemical water quality parameters. Having identified various test formats, the next question is: What do we test for? How can we test for these elements? Fluoride: At least one color disk test kit is available for fluoride. However, portable digital colorimeters are often preferred because of
Learn about how metals produce hydrogen when they react with acid and how hydrogen produces water when it burns with BBC Bitesize. It is also known as anhydrous copper(II) sulfate because it has no water in it.
Read this article to find out where sulfate is found, the importance of testing and how to test for sulfate in water. Sulfate minerals can cause a build-up of scale in water pipes which may be associated with a bitter taste in drinking water.
To test your water quality, start by purchasing a water test kit with strips for testing bacteria, lead, and other markers. Next, fill a glass with room temperature water and dip each strip into the water for 5 seconds, then remove them and shake off the excess.
26, 2019 · Sulfate (SO4) occurs naturally in most of Minnesota's groundwater, with higher levels common in the western part of the state. At high levels, sulfate can give water a bitter or astringent taste and can have laxative effects. This page provides a basic discussion of sulfate in well water and discusses action you can take to minimize it effects.

copper hydroxide reaction ammonium sulphate
