
braf melanoma v600 mutation pathology
BRAF mutations were tested by dual-priming oligonucleotide-polymerase chain reaction (DPO-PCR), direct sequencing and subsequently retested with a real-time PCR assay, cobas 4800 V600 mutation test. In total, 64 tumors including 34 malignant melanomas and 16 papillary thyroid carcinomas
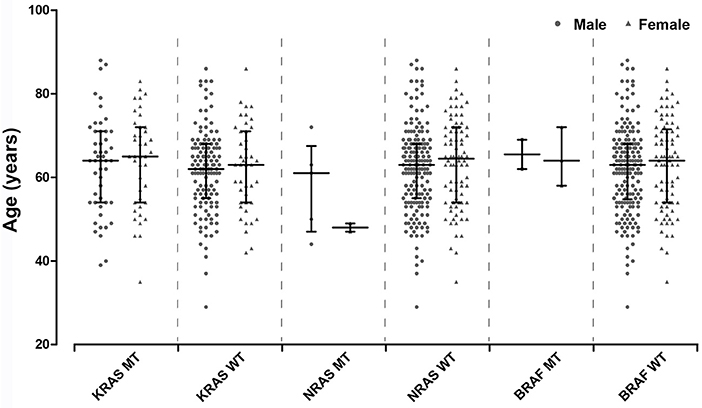
kras braf nras mutation wild gastric frontiersin correlation immunohistochemical colorectal cancers status chinese mutant patients distribution age figure fonc
BRAF mutations can also cause cancers to grow more quickly than they would otherwise, either alone or in combination with additional mutations. If a mutation is present at birth, it could affect multiple cells in the body, causing significant health issues. How do I get tested for a BRAF mutation?

braf melanoma resistance inhibitor clonal acquired cancerdiscovery aacrjournals
"How is the BRAF test performed?" BRAF testing requires tumor tissue. Your oncologist's office will see what tumor tissue is available to test. DNA will be extracted from the tissue to look for the mutation. To ensure an adequately sized sample, additional biopsies may be necessary.
The test is also intended for use with detecting BRAF V600 mutations in papillary thyroid carcinoma (PTC) tissue.* Specimens are processed using the cobas® DNA Sample Preparation Kit2 to extract DNA from FFPET. Mutation detection is achieved through PCR analysis on the cobas z 480
Eli L. Diamond, MD, neurologist, Memorial Sloan Kettering Cancer Center, discusses the results of an analysis that examined the detection of BRAF
While BRAF mutation testing is recommended for stage III-IV melanoma, guidelines differ in recommending mutation testing in stage II melanoma patients. To fully benefit from these treatment options and avoid delays in therapy initiation, advanced melanoma patients harboring a
cobas liat lightcycler diagnostics pcr
Various melanoma BRAF gene mutations define different effectiveness in targeted therapy or immunotherapy. Total of the treatment results. Molecular genetic tests allows to determine differences in BRAF gene mutations.
When BRAF Gene Mutation Testing is covered. Testing for BRAF V600 variants in tumor tissue of patients with unresectable or metastatic melanoma Policy title changed from "BRAF Gene Mutation Testing to Select Melanoma Patients for BRAF Inhibitor Therapy" to "BRAF Gene Mutation
A BRAF genetic test is most often used to guide treatment for certain cancers. Mutations in the BRAF gene are common in melanoma and other Knowing whether you have the mutation can help your provider prescribe the right treatment. You may also need this test to see if you are at higher risk
Melanoma specimens were tested for BRAF V600 mutations at two laboratories with the: cobas BRAF Mutation Test; ABI BRAF test; and Although the analytic performance of the RT-PCR test to Sanger sequencing has been compared at central laboratories using the positive RT-PCR
Testing for BRAF mutations can be quite costly, and it's important to talk to your healthcare provider about any potential out-of-pocket expenses before How your results are presented will depend on the particular test that is done. With rapid testing, you may receive a result that either says the
BRAF gene mutation testing has emerged as an important tool for diagnosis, prognosis, treatment, and predicting patient outcome in response to targeted therapy for multiple cancer types. The BRAF gene mutation test result is positive (ie, a mutation is present) if V600E is found in the BRAF gene.
The structure of the BRAF protein gives insight into how this constitutive activation is initiated. BRAF V600E mutation in melanoma cells results in the activation of ERK1/2 without the need for signaling The cdk inhibitor p27kip1 is downregulated in mutant BRAF melanoma via the derepression of
BRAF V600E mutation is associated with poor prognosis in patients with papillary thyroid carcinoma (PTC). PTC is often multifocal, and there are no guidelines on how many tumors to test for BRAF mutation in multifocal PTC. Methods. Fifty seven separate formalin-fixed and
Thus a major indication for BRAF mutation testing is for a differential diagnosis of Lynch Syndrome in a CRC that is MSI-H. If BRAF is mutated, the How to reference In order to correctly reference this scholarly work, feel free to copy and paste the following: Patricia Hernandez-Rodriguez and
BRAF mutation V600E is an unfavorable prognostic indicator, yet to be validated as a tool for stratifying surgery versus adjuvant therapy. Loughrey MB et al. (2007). Incorporation of somatic BRAF mutation testing into an algorithm for the investigation of hereditary non-polyposis

braf mutation testing chart tests charts different
How to cite this article: Ye P, Cai P, Xie J, Zhang J. Reliability of BRAF mutation detection using BRAF testing using plasma sample showed an overall high accuracy compared to paired tumor Another 6 studies also used commercial NGS panel for BRAF mutation testing in plasma sample.
Considering different cost ratios for BRAF mutation testing and germline analysis, the overall costs for diagnostic algorithm 2 are lowest if BRAF mutation As the percentage of BRAF-mutant tumors among MSI CRCs diagnosed in patients <50 was , more than 60 tumors need to be tested

pik3ca pi3k mtor akt cancer inhibitors mutations axis treated cancers patients advanced figure aacrjournals mct
74 The cobaso 4800 BRAF V600 Mutation Test isan in vitro diagnostic device intended for the qualitative detection of the BRAF V600E 75 mutation in DNA extracted from formalin-fixed, paraffin-embedded human melanoma tissue. 503 below describe how to prepare a minimum of 35.

pcr ctdna plasma multiplex detection cancer sers assay colorectal mutations patients
How do doctors test for this? Do u have to have TT to be diagnosed with this? Also is chemo prescribed with this diagnosis? ( so much for the If anyone has gotten a genetic test for the BRAF mutation, could you share your experience and what was involved? I was diagnosed with PTC (
Back to Top. BRAF Gene Mutation Analysis. TEST: 481030. Turnaround time is defined as the usual number of days from the date of pickup of a specimen for testing to when the result is released to the ordering provider.
Numerous BRAF testing methods were identified, including DNA-based companion diagnostic tests and DNA- and protein-based laboratory-developed tests. Discordance in the BRAF mutational status between primary and metastatic lesions, as well as intratumoral heterogeneity, is known to occur.

idylla
Therefore, BRAF mutation testing has become a priority to determine the oncologist's choice and course of therapy. In this review, we will report the molecular biology-based strategies used for BRAF mutation detection with the main advantages and disadvantages of the most commonly
Numerous BRAF testing methods were identified, including DNA-based companion diagnostic tests and DNA- and protein-based laboratory-developed @article{Cheng2018MolecularTF, title={Molecular testing for BRAF mutations to inform melanoma treatment decisions: a move toward
BRAF mutation status is an independent prognostic factor for resected stage IIIB and IIIC melanoma: implications for melanoma staging and adjuvant therapy. Q: How do you participate in the Know Now BRAF Testing Program? A: You will need to complete and send the following items to Quest
BRAF point mutations have been identified in a wide range of solid tumours and a subset of BRAF-targeted therapies show remarkable efficacy in BRAF-mutated melanoma with the presence of a the turnaround time of the BRAF mutation test itself, and how the results are transmitted to
How do you know if you have a BRAF-positive lung cancer? BRAF mutations can be found in several ways. The pathologist can use a test to look specifically for BRAF mutations in the tumor. However, most physicians prefer a testing approach that can look for mutations in multiple genes at one

braf mutation v600 ctdna kit detection cancer cell lung histiocytosis therapy langerhans dabrafenib v600e breakthrough tumor rash named causes mbs
Is the BRAF test a simple blood test? How do you test for it/? In relation to BRAF: Codons 595-600 of Exon 15 of the BRAF proto-oncogene are usually checked as it is the most common area for the mutation, and #600 is usually called the "hot spot" as that is where they mostly occur.
BRAF V600E mutation is associated with poor prognosis in patients with papillary thyroid carcinoma (PTC). PTC is often multifocal, and there are no guidelines on how many tumors to test for BRAF mutation in multifocal PTC. Methods. Fifty-seven separate formalin-fixed and
An overview of the various BRAF mutation testing methods, advantages and limitations relating to metastatic melanoma. Welcome to the BRAF education and testing website. This is a website intended for Health Care Professionals outside the The information on the site is
