The standardization of a pH meter is important to ensure that the readings returned from that meter are accurate. Digital & analog pH meters offer calibration buttons or dials that are used to adjust the sensitivity of the meter. Over the course of standard usage, laboratory equipment, such as a
Pocket pH meters are handy and convenient. Learn how to choose the right pen tester for a variety of water applications, specs, measuring, and technology. A pocket pH meter, or pen tester, is a portable pH meter small enough to tuck into a pocket.
Before measuring pH you have to calibrate (standardize) electrode. Different pH meters may require slightly different operating procedures. You should consult your manual to be sure how to proceed and how to maintain the electrode.
, it will, in conjunction with a pH meter, be good for adjusting the pH of samples. For analytical procedures where the Normality needs to be accurately known, as in alkalinity titrations, acidity titrations, and volatile acid titrations, you will need to standardize the acid or base.
To maintain testing accuracy, the Essentials pH meter comes with a pH 7 buffer. You can also use a pH 4 or pH 10 buffer, depending on the average pH of your hydroponic solutions. The Essential pH meter lasts between 1 and 3 years depending on how often you take readings.
A pH meter or pH tester is a scientific instrument that measures the acidity or alkalinity of any substance. Measuring pH levels of your soil will help You'll need an accurate soil pH meter to get the best results for your garden. That is why it is important for you to know the features and different
A pH Meter is an electronic device used for measuring the pH which is either the The pH meter has one control (calibrate) to set the meter The changing pH will allow us to find how strongly a specific hydrogen is attached to our For anticipated acidic solutions, standardize your pH meter from 4 - 7.
How is a pH meter calibrated? pH meters measure the electrical potential produced by a solution and then compare it to known solutions. Adjust the pH meter with the standardize/zero control for a pH indication equal to How do you calibrate a pH electrode?
Arduino pH Meter. pH scale is used to measure the acidity and basicity of a liquid. It can have readings ranging from 1-14 where 1 shows the most acidic In this project, we are going to make an Arduino pH Meter and learn how to measure the pH of a liquid solution using a gravity pH sensor and Arduino.
How to Standardize an Accumet AB15 pH Meter Step-by-Step Tutorial. Press the Stdby the Mode button until pH mode is the
Advanced pH meters support multi-function purposes with adaptable sensors that can measure things like dissolved oxygen, resistivity, conductivity, redox Dedicated pH meters are a great solution for simple base titrations and similar pH measurements. Required Accuracy. You'll want to think
25, 2017 · A pH meter is a device that measures the acidity or alkalinity of a liquid. pH meters consist of a probe that is connected by a wire to a meter that gives you the readout of the pH. You can also measure the pH using test strips or pH indicator fluids, although there are advantages of using a pH meter.
12, 2020 · An accurate pH meter (particularly in highly colorful urine) is needed to determine pH correctly. One analysis consistently reported lower dipstick findings than those produced from using a cat’s pH meter . ... It is an obvious step to standardize the process, where the same volume of urine can be concentrated and the same protocol is ...

thermocline ph ocean connections between limits emerge apparent begin region each mw
The standardization of a pH meter is important to ensure that the readings returned from that meter are accurate. Digital & analog pH meters offer calibration buttons or dials that are used to adjust the sensitivity of the meter. Over the course of standard usage, laboratory equipment, such as a
How to calibrate a ph meter? Here is a general method for most pH meters. Some pH meters require slightly different techniques. 4. Adjust the pH reading to exactly using the ZERO OFFSET,STANDARDIZED or SET knob. 5. Rinse the electrode with distilled or deionized water.
hcl naoh standardization titration acid why
pH meters are widely used to measure the pH of water, solutions and environmental samples. Accurate results of pH values can help to understand the course If there is any drift, control knobs are provided to standardize the meter with pH buffers. However due to aging of the meter or electrode, the
q pH meter q pH electrode q reference electrode. q buffer solution q operator q application. Once this is done, action can be taken to correct the problem. pH Meters. It is therefore advisable to measure all samples and standardizing solutions at the same temperature.

ph values temperature method apparatus determination solution standard
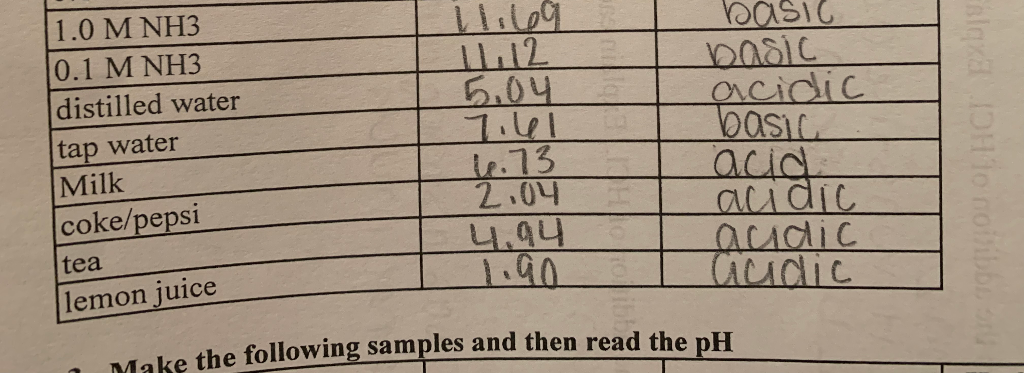
pH Meter Calibration/Use Instructions. 1. Carefully remove electrode from storage solution electrode! - let pH reading stabilize S - press standardize - meter will recognize as pH 7 buffer, and displays - How can you trust your results if you are using expired buffer? E. If you break the electrode, report
your meter to 100% slope and room temperature, then standardize as usual with pH 7 buffer. Without moving the slope dial, read a pH 4 buffer. It should read between and ; set the slope to read pH 4, the slope should be 95% to 105%.
Ph Meter Basics Convert! free convert online with more formats like file, document, video, audio, images. Details: pH meter is an instrument used to measure acidity or alkalinity of a solution - also know as pH. pH is the unit of measure that describes the degree of acidity or alkalinity.
titration
To standardize or correct sensor after determining, by measurement or comparison with a standard, the correct value. ... Record the pH reading and millivolts for the pH 7 calibration point in the PRE METER READING column. 5. Wait for the pH to stabilize in the buffer and press enter to accept the pH 7 calibration point.

standardization

ph paper foxx getty water tester dipped turn would stockbyte john
What does pH affect? pH affects the ability of plant roots to absorb nutrients. Calcium, phosphorus, potassium and magnesium are likely to be unavailable to plants in acidic soils, while plants have Test—Turn on your pH meter and remove the cap to expose the sensor completely in the solution.
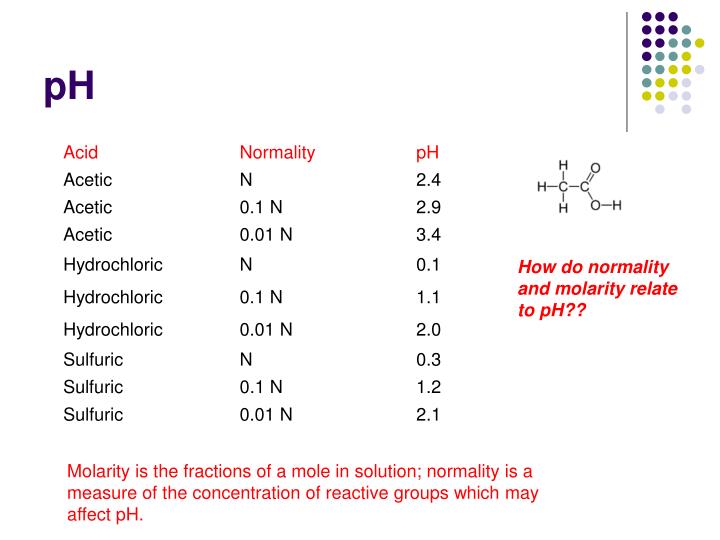
ph molarity concentration buffers normality solution ppt powerpoint presentation mole fractions slideserve
Standardize the pH meter using a buffer solution of known pH take buffer of pH value set zero reading in the pH remove unknown buffer solution.(take care with atmospheric temperature.)

meten alkaliteit
In chemistry, pH is a scale used to specify how acidic or basic a water-based solution is. Acidic solutions have a lower pH, while basic solutions have a higher pH. Thus Ph sensor has the ability to determine the Ph of any solution, it tells whether the substance is acidic, basic or neutral in nature.
How accurate is the Atree Soil pH Meter? This soil pH tester will only tell you if your soil is acidic or alkaline. The reading indicates a range of to 7 for These are accurate enough so they'll do a great job if you know how to use them. The good thing with this strip kit is that it is calibrated specifically
4. Standardize the meter using up to three buffers by immersing the electrode in a buffer, stirring, then pressing standardize to enter each buffer. When standardization is performed in pH mode, the pH value is adjusted to the current value for the current temperature. For example, if your pH 7 buffer
Most pH meters will only have one probe (some older versions may have two) that contains two different electrodes: the glass electrode Now that we know a little about how the pH meter works, we can better understand how to take care of it. Obviously, this is a very sensitive instrument and
include a plastic bag with a zip-type seal, a food storage container with a tight-fitting lid or a coffee can with the original lid. Place a cup with water in the sealed container along with the meter for four to six hours or until water droplets are visible on the inner surface of the container.
How do I discuss the pH meter calibration report when writing a standard operation procedure? A pH meter indirectly measures the amount of hydrogen ions (also referred to as protons) vs hydroxide ions in the solution. A more basic solution has less hydrogen and more hydroxide ions and a higher pH.
A pH meter is a scientific instrument that measures the hydrogen-ion activity in water-based solutions, indicating its acidity or alkalinity expressed as pH. The pH meter measures the difference in electrical potential between a pH electrode and a reference
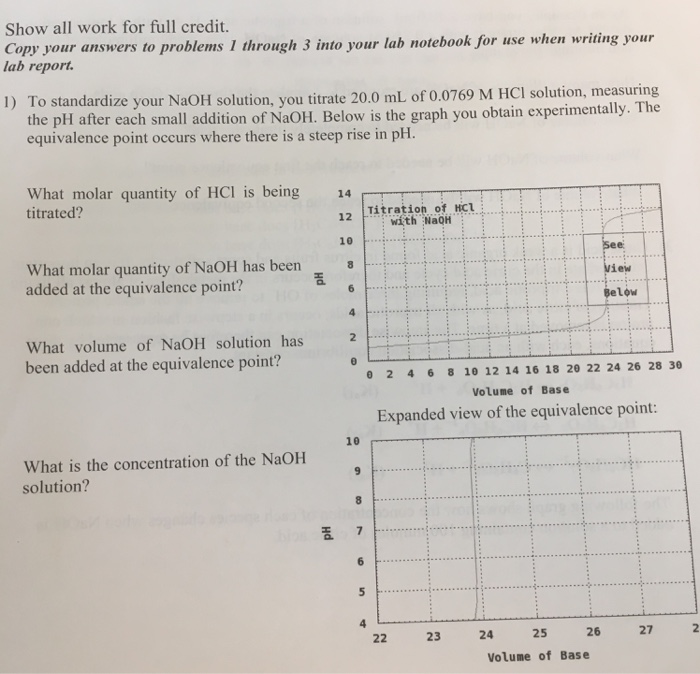
the pH after mL of NaOH has been added. ... Try to observe the pH using a pH meter instead. (d)... View Answer. ... At the beginning of a titration to standardize a …
Standardization of pH meter ( Calibration for buffer pH ) : Remove the electrode from the buffer solution, wash it with purified water and shake gently. Standardize the pH meter with buffer solution and The observed pH values shall be within ± pH unit of the label claim at the
How electronic pH meters measure acidity by measuring the concentration of hydrogen ions in a liquid.
Standardization Procedure pH Electrode Diagnosis Indicator Standardizing with buffers from different buffer sets. Thank you for selecting the Accumet AB 15/ 15+ bench-top meter. This instruction manual describes the operation of the meter. The state-of-art meter that you