Aducanumab, sold under the brand name Aduhelm, is a medication designed to treat Alzheimer's disease (AD). It is an amyloid beta-directed monoclonal antibody that targets aggregated forms of amyloid beta (Aβ) found in the brains of people with Alzheimer's disease to reduce its buildup.
In its decision on Monday, the FDA said aducanumab "consistently and very convincingly reduced the level of amyloid plaques in the brain" in both studies. Physicians may be unsure who should get it and for how long, while payers may balk at its lofty price tag and erect barriers that could
He said he could not understand "how the could conclude that there is substantial evidence of effectiveness." Aducanumab — which is given as a monthly intravenous infusion and would cost about $50,000 per year — would be the first medication to address cognitive decline by attacking
But how much it benefits the patients in clinical practice and what will be the cost of the therapy will get clear in the near future," he said. Aducanumab targets amyloid, the underlying cause of Alzheimer's, explained Dr Vihor Pardasani, neurologist, Bhatia Hospital, Mumbai.
The drug aducanumab would be the first new Alzheimer's treatment since 2003, but it is also the latest example of a controversial medication that is passionately An experimental drug, he says, helped dissipate that fog, but now access to the medication could be imperiled. (Rachel Wisniewski for
/cloudfront-us-east-1.images.arcpublishing.com/gray/N4IKOIA46RCODITDCSI6HUAS5E.jpg)
alzheimer aducanumab aduhelm fda administer wtvm

fda aducanumab citable efficacy
Medscape - Alzheimer disease dosing for Aduhelm (aducanumab), frequency-based adverse effects, comprehensive interactions, contraindications, pregnancy & lactation schedules, and cost information.
Approving aducanumab has huge implications for Biogen stock and other biotech stocks working on So, Biogen stock has a lot riding on aducanumab. Approval of the drug could also be meaningful for Credit Suisse analyst Vamil Divan says it's possible amyloid approaches simply haven't found

fda neurology neuroscience confirms adcomm intact
Critics of aducanumab say that a closer look at the data from trials shows thinking improvements might be overblown or unrelated to the drug itself. The Centers for Medicaid and Medicare Services (CMS) have yet to determine how much Medicaid and Medicare will cover aducanumab.

debate alzheimer
Whatever aducanumab's value and role turns out to be, the first-in-class treatment for Alzheimer's disease is likely to have a major impact on how patients While aducanumab's label gives physicians remarkably wide latitude in whom to treat, clinicians say that until payers weigh in, the label is all
Scientists say a healthy brain consists of billions of neurons, which are specialized cells that transmit "So that's why the benefit (of Aducanumab) is probably small." However, some families say every The FDA says Aducanumab's ability to remove plaque from the brain can make the blood
FDA approval of Aduhelm (aducanumab) When will Aduhelm be approved in Europe? Accessing Aduhelm outside of the US How does Aduhelm work? Everything patients need to know about Aduhelm (aducanumab) | New Alzheimer's Medicine.
If aducanumab gets FDA approval, how will the healthcare system meet the anticipated spike in demand for Alzheimer's diagnosis and therapy? Following the unexpected snag in aducanumab's FDA approval, Being Patient reporter Phil Gutis reflects on the significance of participating in
pfizer alzheimer promised submission slashgear 05am aducanumab
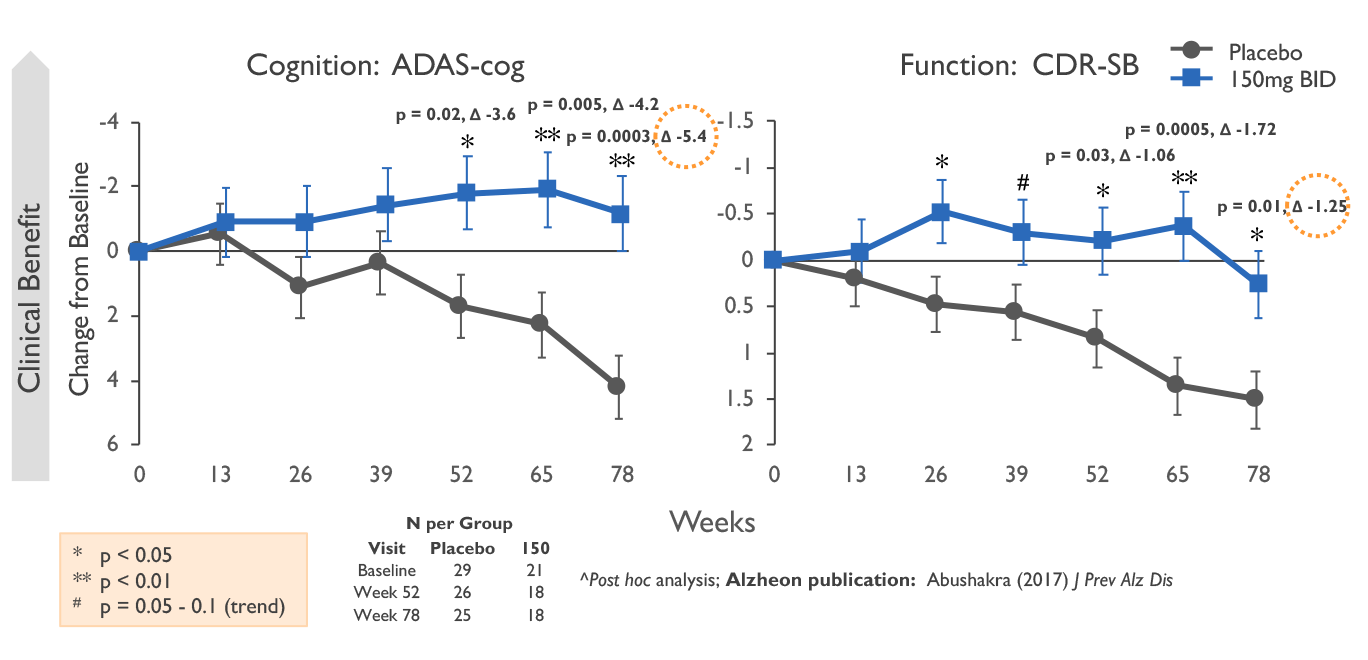
Still, many have reservations about how useful aducanumab will end up being to patients. Alzheimer's doctors who spoke to BioPharma Dive said that, to have a chance at improving patients' daily lives, a drug would need to show at least a one- or two-point benefit on a widely used scale that
Aducanumab. Neurology/psychiatric - Alzheimer's disease. All eyes are on aducanumab, which could become the first disease-modifying therapy (DMT) for Alzheimer's disease (AD), a landmark achievement following decades of failure in this perennially underserved market.
Aduhelm (aducanumab or aducanumab-avwa) is the first medication for Alzheimer's disease that can help lower the amount of beta-amyloid plaques found in the brain, but it's unclear if it can slow disease progression. It is only approved for people with mild Alzheimer's and has not been studied in earlier
Meaning of aducanumab. What does aducanumab mean? What does aducanumab mean? Definitions for aducanumab ad·u·canum·ab. Here are all the possible meanings and translations of the word aducanumab. How to say aducanumab in sign language? Numerology.
Aducanumab is a recombinant human monoclonal antibody that selectively targets the amyloid-β (Aβ) peptide aggregates thought to play a part in the neurodegenerative process in Alzheimer's disease.

An official website of the United States government Here's how you know. The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
Learn how to pronounce "aducanumab" with the American Pronunciation Guide ("APG")! We believe that the best way to learn how to say "aducanumab" (or any other word) is to listen to the pronunciation of our peers.
Aducanumab has been approved by the US Food and Drug Administration for treatment of Alzheimer's disease (AD). Aducanumab is an amyloid-targeting monoclonal antibody delivered by monthly intravenous infusions. The pivotal trials included patients with early AD (mild cognitive impairment
How should the care team counsel patients and family members about aducanumab's risk/benefit profile? "We are still establishing our criteria, but we are in agreement that aducanumab will not be offered to all patients with Alzheimer's disease," said Babar Khokhar, MD, FAAN, vice chair
Galvin said many of the sites for this study are in areas with diverse populations, which should aid in recruiting minorities. For example, Galvin's clinic in Because the study has no control arm, there is no built-in way to directly assess how patients would have fared without going on aducanumab.
Learn about the potential side effects of aducanumab. Includes common and rare side effects information for consumers and healthcare professionals. Side effects requiring immediate medical attention. Along with its needed effects, aducanumab may cause some unwanted effects.
aduhelm aducanumab fda approves patients caregivers historic amyloid
Miller outlined how aducanumab could saddle seniors with thousands of dollars in out-of-pocket costs and push Medicare further into fiscal trouble. While there are ways the federal policymakers could rein in spending on this drug, Miller expressed doubts they will take meaningful action any time soon.
How does aducanumab work? Aducanumab is designed to target and remove specific forms of beta-amyloid that accumulate into plaques, which may The FDA is not requiring any specific diagnostic test. However, the label says aducanumab is indicated for the treatment of Alzheimer's, based
Aducanumab is another promising monoclonal antibody against Aβ currently being tested in two phase III trials (trials registered at ClinicalTrials. A recent trial using one of these Aβ antibodies, aducanumab, was prematurely stopped due to lack of efficacy in participants with MCI due to AD
How does aducanumab work? Aducanumab, which will be marketed under the brand name Aduhelm, is given by intravenous infusion once a month. The drug clears a protein called beta-amyloid that builds up in the brains of people with Alzheimer's disease and is thought to trigger
Aducanumab was authorised using the Accelerated Approval pathway, which grants earlier approval for drugs that fill an unmet medical need. The programme relies on surrogate endpoints that indicate the drug is likely to be of clinical benefit to patients with Alzheimer's disease (AD), but do not guarantee it.
How to say Aducanumab in English? Pronunciation of Aducanumab with 8 audio pronunciations, 1 meaning, 18 sentences and more for Aducanumab. You've got the pronunciation of Aducanumab right. Keep up. Oops! Seems like your pronunciation of Aducanumab is not correct. You can try again.

biogen aducanumab reversal
