
analysis certificates multiv lot
Certificate of Analysis (COA). PharmaState Blog. - 28/05/2019. According to WHO good practices for pharmaceutical quality control laboratories, the CoA lists tests performed on a particular sample with the results obtained and the acceptance criteria applied, followed by an indication of whether or
How to obtain sample certificate of analysis (coa) ? In order to obtain sample certificate of Analysis (CoA), please select "sample CoA" from Certificate Type list.
Define Certificate of Analysis. means a document signed by an authorized representative of Manufacturer, describing Specifications for IDP Portugal shall engage the services of a third party independent laboratory for the purpose of obtaining a Certificate of Analysis for all Project Outputs.
15, 2018 · How to Download a Certificate of Analysis. AVAILABLE IN:English. Last Updated:10/15/18. …

phlebotomy certificate laboratory allied
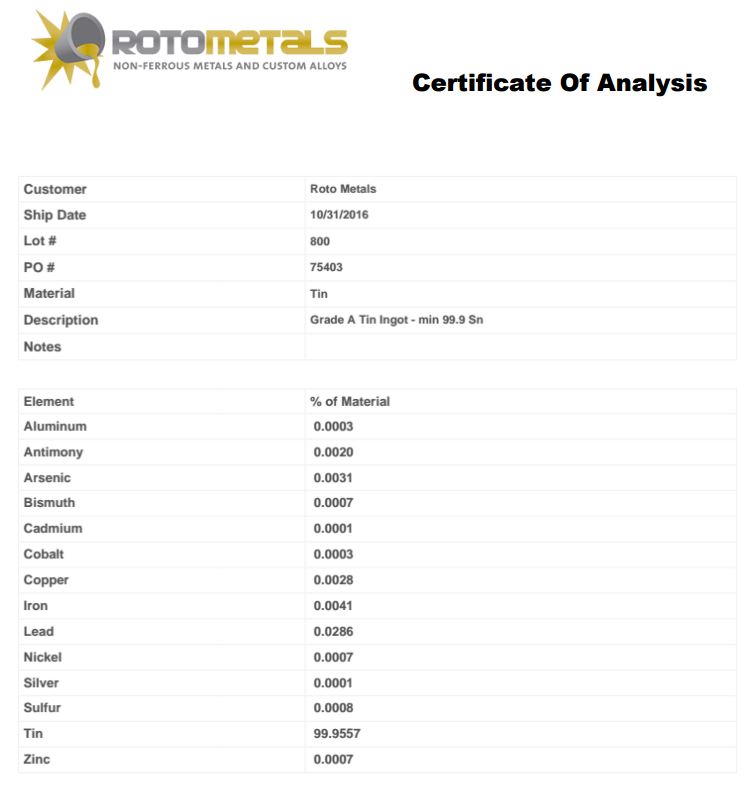
tin certificate analysis ingot pure current
a Certificate of Analysis. Certificate of Analysis Request. The certificate of analysis for that lot is not currently available online. Complete this form to request the certificate of analysis. Account number. Item number. Lot number. Email address. Get a Certificate of Analysis.
A Certificate of Analysis is a document from an accredited laboratory that confirms that a regulated product meets certain specifications. A COA commonly contains the testing results performed as part of the quality control process. Most states with cannabis programs require laboratory testing on
To obtain a medical certificate in the UK, an individual must obtain it from a physician who can attest to the results of a medical examination of a patient. sir please tell me that how we can obtain cast velidation certificate in madhya predash my e mail address is @
When creating Certificates of Analysis to accompany shipments, Quality Control and Quality Assurance departments are very aware of the need to Receiving inspectors will want to examine the Certificate of Analysis before accepting a delivery to confirm that products conform to specifications.
Technical Resource Library. Videos. How to Download a Certificate of Analysis.

certificate analysis purity hemp shipping
certificate of analysis COA A certificate required by some countries as proof of the quality and composition of goods such as pharmaceuticals. certificate of analysis — analizių pažymėjimas statusas T sritis Standartizacija ir metrologija apibrėžtis Dokumentas, kuriame pateikiami išsamūs visų...
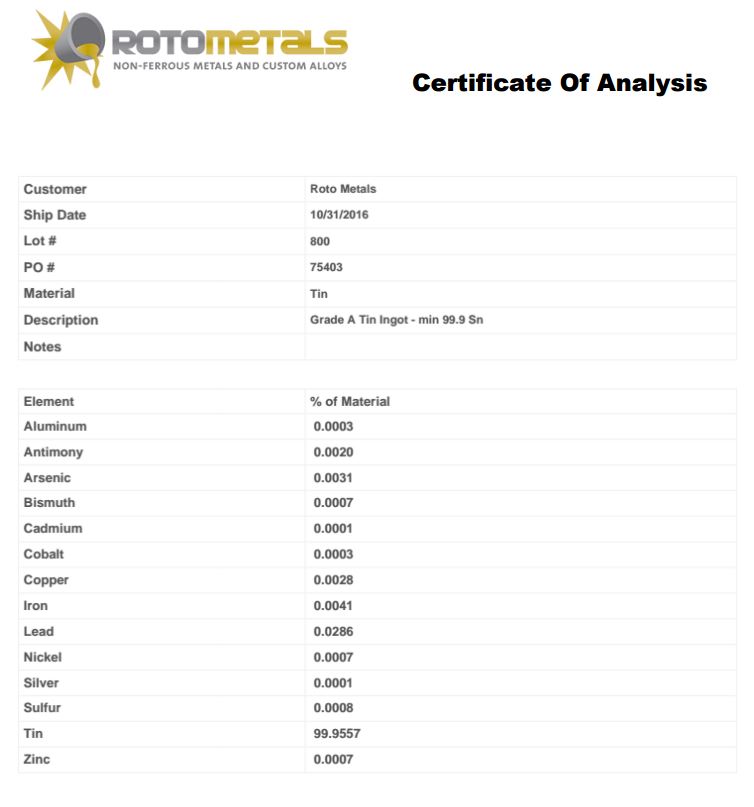
rotometals ingot

20, 2021 · PROCEDURE. Visit Follow the instructions on the screen. For a Column Efficiency Test report provide the last 4 digits of the serial number.
Certificates of Analysis (CoA) have to be issued for excipients, APIs, packaging materials and finished products. Regarding those certificates, there are a number of According to the EU GMP Guide Part I, certificates of analysis provide an overview of test results obtained from a product or a material.
A Certificates of Analysis is essential to provide all the required information about a particular material, confirming that the material is fit for purpose including actual results obtained from testing performed as part of quality control of an individual batch of a product. Please enter your part
can I obtain a Certificate of Analysis (COA)? COAs are available on our website. In order to locate the correct COA for any product, enter the lot number that is printed on the product packaging. If you cannot locate your lot number or have any questions, contact Customer Service.
A Certificate of Analysis, or COA, is a document issued by an accredited laboratory that includes a Here is a little tip on how to make sense of the labels: 100% organic — all ingredients and practices Obtaining a certificate can be divided into five stages: Choose an accredited certifying agent
Restek's Certificates of Analysis, or CofAs, offer comprehensive information to help you understand the Most of our customers use only gravimetric concentration for their analyses. If the results of your analysis do We only display the formula to show how the combined stressed value was calculated.
Back to Main Website. Certificates of Analysis. Please enter a Product Number and Lot Number to download your Certificate
09, 2016 · So a COA or C of A is a ‘Certificate Of Analysis’. Basically it is a piece of paper that gives actual test results for the batch of product that you are exporting. The test results are usually reported against the typical specification. A Specification for a product is a piece of paper that gives guidelines of the physical and maybe chemical parameters of a Reading Time: 9 mins
Certificates of Analysis. What is that piece of paper and what role does it play in GMP compliance? By Paula Brown. might be an online bank glossary, which states: "Certificate of Analysis: A document usually issued by an inspection firm attesting to the quality or purity of exported commodities.
A certificate of analysis (COA) is a document associated with cannabis derived products, attesting to its laboratory analysis for cannabinoids and in some cases adulterants, heavy metals and pesticides, mold, etc.

analysis certificates lot
Why is a Certificate of Analysis prepared and how is it utilized? Certificates of Analysis can be used to satisfy qualification and/or acceptance activities ( receiving inspection) by customers that are subject to regulatory or governing body expectations including, but not limited to the following

science meat certificate tamu agrilife teaching
The Data Analyst Certification, developed by Google, can help you navigate tools and platforms to process, analyze, and visualize data. Get started in the high-growth field of data analytics with a professional certificate from Google. Learn job-ready skills that are in demand, like how to
Business Analysis Certification: What is the leading business analysis certification for Senior level Professionals? What is the Global standard for a Senior Business Analyst Professional? The Globally Recognized Certification for Senior Professionals is a CBAP designation.

fda certificates 510k administration

Certificates of Analysis. Download all your certificates for reference during quality checks and audits. We integrate new technologies into our testing workflow and supplement We use these cookies to collect information about how you interact with our services and to help us measure and improve them.

The Certificate of Analysis is a legally binding document that is issued by a certification authority regarding a product. The document attests that the product has undergone extensive testing in a certified lab. The document will include how the product follows the specifications and
Certificate of Analysis is a document which represents the certified concentration of a component of a mixture. Calgaz Certificates of Analysis require actual analysis and backup data to substantiate concentration and accuracy claims. CofA are now available for download for ALL our customers

multiv
A certificate of analysis is most used certificate to certify the assurance of quality. Merck Certificate of Analysis. Merck is the largest pharmaceutical manufacturing company in the United States and since we know that the pharmaceutical has to be gone through several tests before
The Certificate of Analysis (CofA) provides usage information. To locate the CofA, in the search box on , select the arrow next to "All" and select Certificate of Analysis.
A Certificate of Analysis is a document from our quality control department which provides details regarding the testing that ensures the identity and safety of a botanical that we sell. If you'd like to receive a Certificate of Analysis about a botanical, all you need to do is contact our support
OBJECTIVE: Obtain a copy of CoA and Efficiency Test Report for Waters Columns PROCEDURE: Visit Follow the instructions on the ADDITIONAL INFORMATION. You may contact our Certification Of Analysis team at Certofanalysis@ to request Certification
Our certificates of analysis comply with Amazon and many other retailers requirements. A Certificate of Analysis is a document from a supplier that states the identity, purity or microbiological state of a product. It shows that the supplier completed the required testing and that the results
Leader in Analytical Reference Standards. Certificate of Analysis. Multi-component organic standard sample. Certification meets the requirements outlined in ISO Guide 34.

nursing teacher classes vs lpn degree nurses take which rn certificate license career better physicians hospitals offices schools

analysis certificates lot
of Analysis . Standard Reference Material® 1635a. Trace Elements in Coal (Subbituminous) This Standard Reference Material (SRM) is intended primarily for the evaluation of techniques used in the analysis of coals and materials of a similar matrix. A unit of SRM 1635a consists of 50g of subbituminous coal that was

meja checkpoint
Learn Technical Analysis to identify trading opportunities with the help of charts, patterns, support In this course you will be able to learn what is technical analysis of stocks & how to predict future movements Students enrolling for Certification in Online Technical Analysis course will be given

tensorflow keras
How can we improve this answer? If you need additional assistance, please leave us your email address. Why do the concentrations of the highest calibrators in the certificate of analysis (CoA) for validated kits vary between kit lots?
For the issue of a certificate of analysis you can usefully refer to the requirements of paragraph of the EN ISO/IEC 17025:2005 standard. This is a document signed by a competent analyst that includes the product name, list of ingredients, product batch number, the analysis, the method

