Corrosion—the deterioration of engineering materials by chemical interaction with their environment—is an extraordinarily costly problem. Damage to unchecked corrosion on tubing systems is a leading cause of profit loss for offshore and nearshore applications, costing billions each year.
Undercutting sideway pitting corrosion cross section diagram. How to test for Pitting Corrosion? Electrochemical testing to measure pitting in any metal such as cyclic polarisation and How to treat Pitting Corrosion? It is bed to use recommended cleaning procedures to expose the pits fully
The pitting is likely the best known corrosion phenomena in relation to stainless steels. But what causes this phenomenon? And how can you avoid it? The pitting corrosion is one of the most insidious forms of corrosion. This may cause breakage by drilling.
21, 2016 · Pitting is a highly localized form of corrosion, resulting in the development of cavities or pits. This corrosion is much less predictable, and usually occurs in metals where the composition is not uniform. Pitting can be reduced with the use of a surface finish.

radiography rt digital detector array dda ndt
30, 2020 · Non-uniform corrosion, which is the isolated development of corrosion cells across the inner surface of a pipe wall. Excessive pitting corrosion can lead to pinhole leaks in the pipe and result in water damage and mold growth. …

corrosion h2s electronic pollution intechopen metals figure
Why Is Corrosion Monitoring Important? How Do You Perform Corrosion Monitoring? Corrosion often first appears as a discontinuity in a material, such as a discoloration or some other Monitoring hydrogen penetration . Using probes, you can measure the quantity of hydrogen that dissolves
Pit morphology on sensitized 310S stainless steel has been studied using an image processing method based on reflected light microscopy. Salt Spray (fog) test has been used to induce the pitting corrosion. Morphological pits characters do not depend on sensitization heat treatments applied here.
How does galvanic corrosion and pitting corrosion occur? Pitting corrosion - Mechanism At anode the metal gets oxidised to liberate electrons. You will be able to suggest the preventive measures of pitting corrosion.
Pitting corrosion is a form of localized corrosion, which produces attacks in the form of spots or pits. Pitting corrosion attacks most often take place at points where the passive layer might be weakened, for example by slag inclusions, a damaged surface or imperfections in the passive layer.
corrosion experiments focus on particular processes (general corrosion, pitting corrosion, etc.) and consider a number of parameters such as temperature, solution pH, oxic/anoxic conditions, concentrations of species such as Cl-and sulphur, and radiation field. The surface finishing of the metal sample is usually an important step in ...

corrosion probes coupons probe monitoring internal types

Pitting Corrosion. Passive metals, such as stainless steel, resist corrosive media and can perform well over long periods of time. Pitting corrosion can lead to unexpected catastrophic system failure. The split tubing above left was caused by pitting corrosion of stainless steel.
Many materials are susceptible to pitting corrosion, but the important thing is the service conditions. For measuring the pitting potential of any metal you can use the cyclic polarisation and potentiostatic test. these are the short term electrochemical tests and give the instant results.
01, 2017 · Corrosion films affect the exposed area, and reaction rates even near E corr can be coverage dependent . For these reasons, instant corrosion rate determinations using PDP should be used with caution and their combination with other electrochemical and non-electrochemical techniques, , EIS and hydrogen collection, is usually recommended.
Home » Knowledge Hub » How to measure pitting corrosion. There are many different approaches to the measurement of corrosion, and specifically pitting corrosion. A number of the industry-standard tests for corrosion, such ASTM G28 and ASTM G48, exist.
Definition and Examples of Corrosion ... • SCC and pitting of stainless steel in sea water The Need to Control Corrosion ... it is necessary to infer possible mechanisms from indirect measure-ments and observations. Examples are the rate of change in weight or
This phenomenon is called pitting corrosion or simply pitting. You can measure a logarithmic current versus potential curve over a range of about one half volt. How is corrosion current used to generate a corrosion rate? Assume an electrolytic dissolution reaction involving a chemical species, S
Pitting corrosion is an electrochemical oxidation-reduction (redox) process, which occurs within localized holes (cells) on the surface of metals coated with a passive film. Pitting corrosion is considered much more dangerous than uniform corrosion since its rate is 10-100 times higher.
RE: Measuring pitting. rustbuster1(Materials) 27 Mar 02 14:34. The cheapest method is to have a wrap-on repair kit available and wait until the pipe Examine how the principles of DfAM upend many of the long-standing rules around manufacturability - allowing engineers and designers to place
phenomenon is called pitting corrosion or simply pitting. Because corrosion occurs via electrochemical reactions, electrochemical techniques are ideal for the study of the corrosion processes. In electrochemical studies, a metal sample with a surface area of a few square centimeters is used to model the metal in a corroding system.
Pitting corrosion, or pitting, is a form of extremely localized corrosion that leads to the creation of small holes in the metal. The driving power for pitting corrosion is the depassivation of a small area, which becomes anodic (oxidation reaction)...
Localized corrosion such as pitting and crevice corrosion of stainless steels generally occurs in the presence of halide ions, typically chloride ( coastal and deicing chloride salts - sodium, calcium or magnesium chlorides; hydrochloric acid; bleach - sodium or calcium hypochlorite; and other
What materials are susceptible to pitting corrosion? Pitting corrosion is usually found on passive metals and alloys such aluminium alloys, stainless steels and stainless alloys when the ultra-thin passive film (oxide film) is chemically or mechanically damaged and does not immediately re-passivate.

turbine blade prediction pitting corrosion geothermal useful damage
Pitting corrosion can have serious implications because the cavity it bores below the surface can be both wide Pitting corrosion, on the other hand, causes pits that measure in millimeters in depth. How to Fix Pitted Surfaces. Corroded surfaces and pits should be thoroughly cleaned to render
A brief graphical overview on how to determine pitting and repassivation potentials from real anodic polarization curves. All about corrosion science and engineering. Privacy & Cookies: This site uses cookies. By continuing to use this website, you agree to their use.
Modelling Pitting Corrosion in Carbon Steel Materials. The Concept of Pitting Corrosion To understand pitting of carbon steel, one must first comprehend the. Figure showing a pit gauge measuring a pit of depth millimetre. Numerous of previous work stated that there are three
4. How to assess corrosion in an specific environment/application. 31. Factors influencing 3, we are able to explain many forms of corrosion and also to deduct measures to mitigate corrosion. Pitting corrosion is a localized form of corrosion that leads to the creation of small holes or "pits"
3. Corrosion • General Corrosion • Pitting Corrosion • Crevice Corrosion • Stress Corrosion Cracking • Galvanic Corrosion • Microbiologically Influenced 4. 4 Pitting and Crevice corrosion are forms of localised corrosion, which means that the corrosion occurs in a limited area on the pipe.

corrosion aircraft controlling preventing metal objects exclusive
There are a variety of methods for measuring pit depth. Ultrasonically, mechanically, etc. wow nice information regarding the can measure the deapth of pitting corrosion.

cavitation erosion
flux leakage (TFI or Transverse Field Inspection technology) is a magnetic method of nondestructive testing that is used to detect corrosion and pitting in steel structures, most commonly pipelines and storage tanks. The basic principle is that a powerful magnet is used to magnetize the steel. At areas where there is corrosion or missing metal, the magnetic field …
17, 2014 · However, the susceptibility of aluminum to pitting depends on many factors, such as chloride ion concentration, pH, dissolved oxygen in the corrosion environment and surface condition. 2 Furthermore, aluminum alloys themselves can contribute to pitting problems due to preferential etching.
Pitting corrosion is quantified in different ways average pit depth, maximum pit depth, volume etc. Importance of 3D non contact profilometer for In this application the Nanovea ST400 Profilometer is used to measure the surface of a corrosion pitted stainless steel coupon.
Pitting corrosion in metals can be attributed to neutral to acidic soultions containing halides ,primary The corrosion itself IE pitting cannot be reversed. A treatment would consist of neutralization and cleaning the affected area then an application of a coating that would repel
Pitting Corrosion: This type of corrosion causes tiny pits to form along the length of the metal pipe, thus the term pitting corrosion. Such corrosion diminishes the pipe's wall thickness, reducing its strength and pressure bearing capabilities. Crevice Corrosion: A bigger cause for concern,
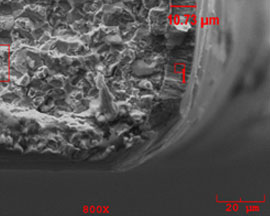
corrosion zinc plating coatings scanning electron microscopy
pitting resistance of an austenitic stainless steel can be related directly to alloy composition, where chromium, molybdenum and nitrogen are a weight %. The Pitting Resistance Equivalent Number (PREN) uses the following formula to measure an alloy's relative pitting resistance - the higher the number, the better the pitting resistance.
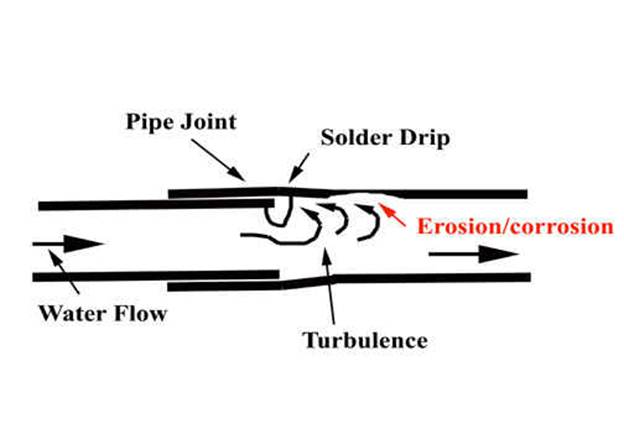
corrosion erosion pipe copper water mechanism velocity line internal soldering pex service solder local breakdown causing galvanized steel failure figure
Pitting corrosion is localized accelerated dissolution of metal that occurs as a result of a breakdown of the otherwise protective passive film on the metal surface. The phenomenology of pitting corrosion is discussed, including the effects of alloy composition, environment, potential, and temperature.
Pitting corrosion is a localized form of corrosion by which cavities or "holes" are produced in the material. Pitting is considered to be more dangerous than uniform corrosion damage because it is more difficult to detect, predict and design against.
tools,corrosoion rate calculator, weight loss, area, density, time, specify units, mm, gm, days, hours, seconds, cc, calculate, millisec
Pitting corrosion can be evaluated and ranked using the CPT in accordance to the ASTM Standard G48-03 [25] . Figure . Current density versus temperature to measure critical pitting temperature at °C/min temperature. DSS 2205 metallurgy was used with several concentrations
Pitting Corrosion is the form of corrosion that particularly occurs at one spot. We can call it The depth of the corrosion is measured using a calibrated microscope. In this article, we will discuss what is pitting corrosion, its mechanism, test, damages caused by pitting corrosion, and how
28 4 How to assess corrosion in a specific environment and application. 28 Factors influencing atmospheric corrosion 30 Assessment of 3, we are able to explain many forms of corrosion and also to put measures in place to help mitigate it. By mitigating these partial reactions, the
