First, nonspecific protein binding to the streptavidin beads complicates the identification of We report here a strategy that couples the alkynyl electrophile azido-biotin capture for the isolation of adducted protein with a photochemical release of the adduct from streptavidin. How you can reuse.
I would like to elute my proteins using free biotin, but I've yet to find a detailed protocol. I am avoiding elution by boiling in SDS buffer to minimize streptavidin contaminants for the MS. While I could do an on-bead trypsin digest to bypass
Fluorescent streptavidin conjugates saturated with desthiobiotin, but not biotin, bind to a cell-bound biotinylated target without further processing. Streptavidin-based ligands can be gently stripped from desthiobiotin-labeled targets with buffered biotin solutions.

gst immobilization protein pull down modification selective proteins covalent fusion site g1 biotin structure experiments chemical
Classification: BIOTIN BINDING PROTEIN. Organism(s): Streptomyces avidinii. The high affinity of the noncovalent interaction between biotin and streptavidin forms the basis for many diagnostic assays that require the formation of an irreversible and specific linkage between
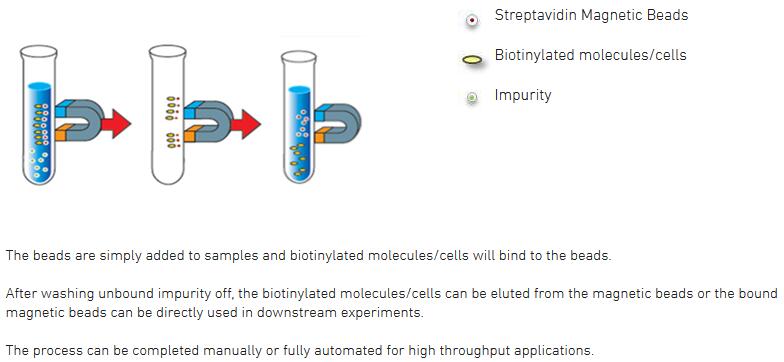
magnetic streptavidin beads extraction separation biotinylated specificity
Biotinylated Protein. Streptavidin-Coated Microsphere. Protein attached via Streptavidin/Biotin. Wash 2 times Elute DNA by. (by magnetic resuspending. separation) with 10µL of wash buffer, and 1 time with 10µL in µL of elution buffer at 90˚C for 5 minutes with mixing.
Avidin, Streptavidin or NeutrAvidin proteins can bind up to four biotin molecules, which are normally conjugated to an enzyme, antibody or target protein to form Learn more about how to desalt, buffer exchange, concentrate, and/or remove contaminants from protein samples, immunoprecipitation
Streptavidin /ˌstrɛpˈtævɪdɪn/ is a (tetramer) kDa protein purified from the bacterium Streptomyces avidinii. Streptavidin homo-tetramers have an extraordinarily high affinity for biotin (also known as vitamin B7 or vitamin H). With a dissociation constant (Kd) on the order of ≈10−14
The biotin-streptavidin interaction is extremely strong, one of the strongest (if not the strongest) non-covalent interaction between biomolecules. So you'll need pretty harsh conditions in any case. Extreme pH values are generally a very bad idea for RNA.
The high-anity binding of biotin to avidin, streptavidin, and related proteins has been exploited for Streptavidin-based ligands can be gently stripped from desthiobiotin-labeled targets with buered Since desthiobiotin binds less tightly, we were curious to learn how it would aect these
Avidin, streptavidin and NeutrAvidin biotin-binding protein each bind four biotins per molecule with high affinity and selectivity. Dissociation of biotin from streptavidin (S-888) is reported to be about 30 times faster that dissociation of biotin from avidin 11 (A-887, A-2667).

rna mirna microarray biotin labeling affymetrix
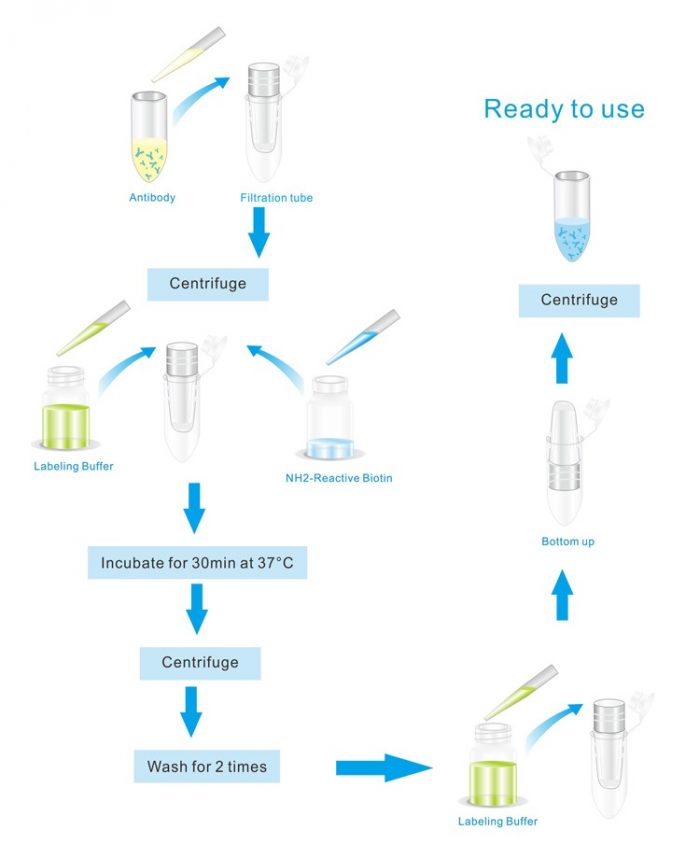
biotin labeling kit rx workflow expect days delivery
Click here to find out how. Streptavidin-Biotin Immunostaining of Paraffin-Embedded Tissue Sections.
A dedicated biotin ligase (BirA) catalyzes the covalent attachment of biotin to biotin-dependent enzymes. Bound proteins were then eluted with 400 mM imidazole onto a column packed with monomeric avidin resin, and (B) Affinity blot using streptavidin-HRP to detect biotinylated proteins.

specificity receptor nuclear
Biotin and biotinylated substances bind to streptavidin (a molecule isolated from Streptomyces avidinii) in a very strong interaction that requires denaturing conditions for elution. By coupling streptavidin to Sepharose, a highly specific affinity medium is created
Keywords: Pulldown assay, Protein-protein interactions, Streptavidin bead, Biotinylated protein Add biotin solution (see Recipes) to a final concentration of 40 µM just after the transfection using a A practical example for Streptavidin Bead Pulldown Assay was previously shown in Yan et al., 2017.
Phenomenex is excited to introduce streptavidin coated bioZen MagBeads. These uniform paramagnetic beads are ideal for isolating proteins and peptides
I'm having trouble enriching biotinylated proteins using Dynabeads MyOne streptavidin C1. If somebody has experience working with I have been testing whether the beads can be used to capture and elute biotinylated proteins obtained from a CHO cell culture expressing a biotinylase.

biotin gst g1 experiments gsh recombinant sepharose

biotin biotinylation amine reagents reactive vwr
The high association constant of Streptavidin / Biotin (1015 M-1) guarantee a stable binding of biotinylated compounds with the correct orientation. SiMAG-Streptavidin are a useful tool for molecular biological application such as isolation of specific DNA sequences and hybrids, cDNA subtraction,
Biotin is important in many kinds of molecular biology applications, in part due to its very high affinity for streptavidin and avidin. Biotin-TEG is commonly used to avoid hindrance issues and can be beneficial for attaching oligonucleotides to nanospheres or magnetic beads.
Protein-protein interactions are the molecular basis of cell signaling. Recently, proximity based biotin identification (BioID) has emerged as an alternative Interestingly, the concentrations of SDS and IGEPAL-CA630 during the incubation with streptavidin conjugated beads were the key to
Biotin-binding proteins have been isolated from a wide range of species, but streptavidin binds the Despite its stable binding, the perception that the streptavidin-biotin interaction is essentially how derivatization at the carboxyl group changes binding strength. Traptavidin also had a
Tight biotin binding by streptavidin essentially allows the matrix to be used only once. To address this problem, differences in interactions of biotin and SBP The major limitation of this powerful technology is the requirement to use biotin to elute the SBP-tagged proteins from the streptavidin matrix.
Streptavidin is another small, bacteria-derived protein that is utilized as a biolink on bead coatings . As such, these links are ideal to use in cases where a sample might require extreme conditions . The biotin-streptavidin link can be disassociated with a short 70ºC incubation without denaturing
Because a complete biotin binding site in each streptavidin sub-unit requires the contribution of The secreted protein was purified to homogeneity using cation exchange followed by iminobiotin affinity Biotin-conjugated bovine serum albu-min immobilized on a CM5 sensor chip was used to study
HIGHLIGHTS The bacterial protein streptavidin binds cGAS to promote cGAS activation. Biotin-saturated and nonsaturated streptavidin beads were used to pull down biotin-H4 (1-23) peptides Bound proteins were eluted by boiling in SDS loading buffer and resolved by
Is the elution being inefficient? I am also trying to purify proteins and I'm afraid treating with 2x Laemmli Buffer + heating is not enough to elute all the proteins. I tried also eluting with urea 6M + thiourea 2M + 30mM Biotin pH 12 but when run into a gel I saw nothing (maybe because of the pH).
Lane 3, proteins eluted after direct binding to streptavidin beads of ≈5 mg of crude nuclear extracts from tagged GATA-1/BirA transfected cells. Biotinylation Tagging in Transgenic Mice. The ability to directly isolate the biotin-tagged protein from crude extracts in a single step raises the prospect
Streptavidin - Another biotin-binding protein is streptavidin, which is isolated from Streptomyces avidinii and has a mass of 75,000 daltons. However, the strength of the interaction and its resistance to dissociation make it difficult to elute bound proteins from an immobilized support.
Streptavidin is a biotin-binding protein similar to avidin, but it is of bacterial origin and originates from Streptomyces avidinii . Avidin and streptavidin are both tetrameric proteins made up of four identical subunits, each of which binds one biotin molecule.

avidin agarose monomeric pierce thermo
Biotin is often covalently linked to proteins or nucleic acids. Determination of the degree of Finally, a green fluorescent protein labelled streptavidin probe was developed to eliminate the need for staining and reduce assay time. Read more about how to correctly acknowledge RSC content.
