Read the International Chemical Safety Card (ICI) for the acid. You should not throw away acids in the garbage or the sewage system as it can have dangerous consequences.[1] X Expert Source Gregory Cade, JD Environmental & Occupational Law Attorney Expert Interview. 13 October 2021. The ICI tells you all the important safety information about handli…Wear proper protective equipment (PPE). When handling acids or any other strong chemical…See all 4 steps on (82)Views: 109KEstimated Reading Time: 9 minsPublished: May 24, 2006

acid nitric bottle ph hazard symbol acs reag eur analysis sigma
19, 2012 · Decontamination/Waste Disposal Procedure Spills may be neutralized with sodium bicarbonate or baking soda. Do not dispose of HCl by pouring down drains followed by copious amounts of water without neturalization. General hazardous waste …
Hydrochloric acid is a clear, colorless, highly pungent aqueous solution of hydrogen chloride (HCl) in water. It is a highly corrosive strong acid, an essential reagent for any amateur chemist. Physical properties of HCl, such as density,
02, 2017 · You can neutralise the acid by mixing with alkali. However, be careful. You will need quite a lot of something like baking powder. Or if you have any left over - cement powder. Add a small amount to the acid - it will foam and release gas (Carbon Dioxide). Don't add too much at once or the bucket will overflow and you will get acid : 2
Hydrochloric acid is a highly corrosive, strong inorganic or mineral acid found in most laboratories. If not stored and handled properly, this can pose a serious threat to the health and safety of laboratory personnel, emergency responders and chemical waste handlers.
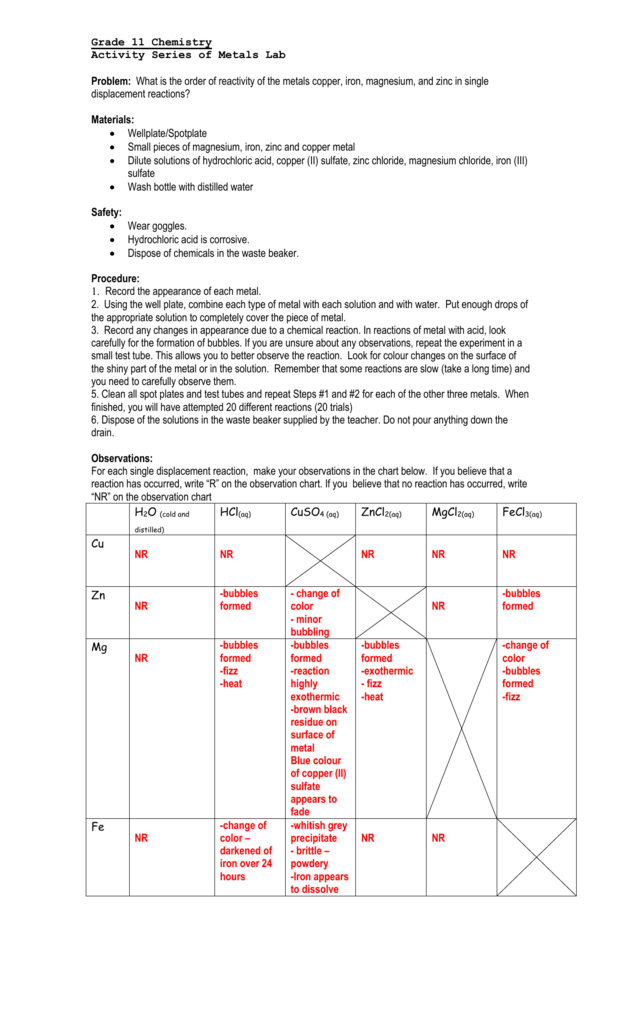
activity metals lab chemistry reactivity copper iron data order grade

acid hydrochloric solution hydroxide sodium bases acids turmeric salts liquids given three cbse ncert third another indicator
Hydrochloric acid is highly corrosive and its concentrated form usually releases an acidic mist that is quite dangerous. To avoid these situations, you need to know how to dispose of muriatic acid safely and thus I want to give you a step by step guide of how to go about it.
Hydrochloric acid. Choose appropriately sized containers. Store smaller quantities in smaller containers. It's not cost effective to dispose of 50 milliliters of material in a 4-liter container.
The proton produced by the dissociation of hydrochloric acid protonates the alcohol molecule in an acid-base reaction. Problems arise with the Arrhenius theory, however. One problem involves the ion that is responsible for acidity H+. Look again at the equation for the dissociation of hydrochloric acid.
29, 2016 · In this video I show you guys how to properly dispose of your excess HCL from experiments.
Acid washing your concrete or your pool walls is another way to dispose of or use up leftover muriatic acid or hydrochloric acid. Final Thoughts. Now you know how to safely dispose of your muriatic acid. By using simple household items, you can neutralize the acid and dispose of it safely yourself.

acid hydrochloric solution standardisation using advertisement sodium carbonate
and organic acid: Inorganic acids, hydrochloric acid, sulfuric acid, phosphoric acid. Organic acids, acetic acid, citric acid, butyric acid; Base. ... waste is still hazardous and is sent for disposal or treatment by one of the other methods described here).

permanganate potassium dispose written july dangerous
Hydrochloric acid is a simple diatomic molecule consisting of a hydrogen atom and a A solution of hydrogen chloride in water is known as hydrochloric acid. Its chemical formula is HCl It has a unique pungent smell
Hydrochloric Acid (HCl) easily dissolves in Water because both Hydrochloric Acid (HCl) and Water (H2O) are Polar Covalent Molecules. This makes the entire HCl and H2O molecules polarized which creates a net dipole moment in each of the molecules as shown below
11, 2021 · Fill a glass or acid-resistant plastic container with 3 gallons of water. Slowly pour 1/4 cup of muriatic acid into the water. Never add the water to the chemical, as any splashing can cause injury. Slowly and carefully pour the diluted solution down the drain.
hydrochloric ozbargain

acid acetic beaker solution treatment suspension usgs allow container another liquid ml pour wash supernatant waste sample carbonates remove until
10, 2014 · Hydrochloric Acid Storage and Disposal. Hydrochloric acid should be stored in a cool, dry, well-ventilated area away from sources of moisture. Keep away from incompatible materials such as oxidizing agents, organic materials, metals and alkalis. Hydrochloric acid has the ability to corrode metallic surfaces.
Due to how incredibly corrosive this acid is, it has proven to be highly effective at keeping home pools clean and properly maintained. As for the eyes, hydrochloric acid will do permanent damage and may even cause blindness. It's also important that you don't inhale the fumes of hydrochloric acid.
03, 2022 · How to Dispose of Muriatic Acid Safely Things You Wiall Require. Step By Step Instructions. As we said before, hydrochloric acid is highly corrosive and its concentrated form Things to Remember. This is effervescent and exothermic, mix slowly and carefully, adding sodium to the acid NOT ...

hydrochloric dilute

acid hydrochloric neutralizing
Hydrochloric acid (also called HCL, HCL acid or betaine hydrochloride in supplement form) is considered one of the most important fluids (or "juices") found in the human body. Read on to learn more about the benefits of hydrochloric acid, how to improve its production and more.
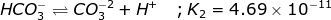
acid ionization constant equation neutralization process math problems carbonic
Hydrochloric acid, also known as muriatic acid, is generally used to clean concrete foundations or patios. Dispose of the etchant properly or store the solution in a glass or plastic container with a lid. Mark the contents of the container and store it in a responsible place out of heat and excess light

acid safely dispose wikihow
In this tutorial I set up a burette and use a pipette to load 10 cm3 of a 2 Molar solution of Hydrochloric acid into a conical flask. Then I fill
The chemical compound hydrochloric acid (or muriatic acid) is the aqueous (water-based) solution of hydrogen chloride gas (HCl). This strong acid is highly corrosive and must be handled with appropriate safety precautions. It is the major component of gastric acid.
I've got a gallon of HCl I'd like to dispose of responsibly. I know I can neutralize it with baking soda, but how much and is that practical? In synthesis of aspirin, why does H+ attack the carbonyl group of the anhydride instead of the hydroxyl group of salicylic acid?
~ to the exothermic nature of the reaction, dilution of hydrochloric acid has to be done by slowly adding the acid to water to limit the risk of splashing concentrated acid out. Hydrochloric acid solutions have to be disposed as a hazardous waste in the appropriate acidic waste container. NOTE: Any deviation from this SOP requires approval from PI
How do you dispose hydrochloric acid? Pour it down the drain and then run water over. Make sure that it is diluted. Hydrochloric acid, HCl, is a strong acid.

acid hydrochloric uses common cleaning household aluminium ehow many safely produced applications mind website
Find Out How To Dispose Of Muriatic Acid in 2021. See When The Damage Is irreversible And Can Lead Muriatic acid, also known as hydrochloric acid, is one of the common household products you can use to clean a swimming pool.

acid hydrochloric ghs signs sign concentrated chemical symbols safety would seton each
Hydrochloric acid has many uses. It is used in the production of chlorides, fertilizers, and dyes, in electroplating, and in the photographic, textile, and rubber industries. Hydrochloric acid is corrosive to the eyes, skin, and mucous membranes. Acute (short-term) inhalation exposure may cause eye,

acid hydrochloric distribution larger cleaning abl abldistribution

acid bad breaking hydrofluoric bathtub chemistry episode mike walt firepower ones killed todd its dissolved dissolve tokyo bodies حمض boy
Hydrochloric acid (HCl) will remove the chrome quickly but will not touch the nickel plating, so the effort will not fix your poorly plated part or make it You can make a small quantity of hydrochloric acid on laboratory scale from ingredients like sulfuric acid and salt -- but then you need to ask how
Concentrated hydrochloric acid (HCl) is the most frequently used halogen acid for the dissolution of geologic samples. Hydrochloric acid can be effectively removed from sample solutions by repeated evaporation to incipient dryness with HNO 3 because the boiling point of the HCl azeotrope (110 °C)...
Hydrochloric acid reactions consist of common strong acids, for example, hydrogen gas is displaced in metal reactions, reactions in which hydroxide and metal oxides become neutral with the formation of metal chloride and water, reactions consisting of weak acid salts in which the displacement of

hydrochloric dilute
Hydrochloric acid Hydrochloric acid IUPAC name Hydrochloric acid Other names Muriatic acid, Spirit of How to Quickly Check Pipettes? Correct Test Weight Handling Guide: 12 Practical Tips. The physical properties of hydrochloric acid, such as boiling and melting points, density, and
I have some very old bottles of muriatic/hydrochloric acid that I need to get to the dump. I was planning to pack them in a cardboard box with paper packed Then your mission to dispose of the acid has failed, and to add insult to injury, you've just lost a battle of wits with a sanitation specialist.

synthesis acid nitric lab experiment skin caustic concentrated burn caution crystallization solid multistep extremely exposed cold experiments
But while hydrochloric acid contains only HCI molecules, muriatic acid is made up of HCI molecules as well as impurities such as iron. Due to all of the damage this chemical could cause in one wrong move, extreme caution is mandatory when using, storing, and disposing of muriatic acid.
HCl can be combined with baking soda to form salt water and CO2. NaHCO3 + HCl = NaCl + H2O + CO2. NOTE this is exothermic and effervescent, mix slowly, adding the soda to the acid. In a wide-mouth container.

hydrochloric
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid.
To fully grasp how hydrochloric acid works, it's helpful to understand how digestion works, too. Hydrochloric acid is a primary component of your stomach acid, accounting for percent of its total volume; however, that's not the only important substance.
This a tutorial on how to make HCI by mixing salt and vinegar. I found this as a thing to make shiny pennies it uses 4 solutions number 4# is HCI. Science Concept: By mixing vinegar and salt to make hydrochloric
Never dispose of hydrochloric acids or any other acids or bases on the ground, in a storm drain or in a gutter, because they can contaminate groundwater If you are unsure about how to dispose of hydrochloric acid correctly, ask your local waste facility for advice. Some towns have
It is important to dispose of acids with very low pH (<2) safely. If the acid doesn't have heavy metals or other toxic substances dissolved in it, neutralizing the pH to a less acidic level To determine how much neutralizing solution you'll need, you must know the pH of the acid you're trying to neutralize.
SULFURIC ACID - dehydrates concentrated hydrochloric acid to release some 250 volumes of hydrogen chloride gas. Collect and reclaim or dispose in sealed containers at licensed waste disposal site. This material and its container must be disposed of as hazardous waste.
acid, also known as muriatic acid, the water-based solution of hydrogen chloride, is a highly corrosive acid. It is used to make batteries and fireworks, make gelatin and process sugar, but it is also produced naturally in the stomach to aid digestion as gastric acid. You must dispose of hydrochloric acid as hazardous …PreventionPreparationYour state may allow you to pour diluted hydrochloric acid down your sink. Ensure the room is ventilated by opening windows and doors. Cover all areas of your skin with suitable protective clothing, such as long sleeves, safety goggles, a mask and rubber or neopren…See more on : Claire GillespiePublished: Mar 14, 2018
