Electrolysis of seawater in a mercury cell leads to the production of chlorine and sodium hydroxide at the same time. This method involves using mercury as the cathode and graphite as the anode. The mercury attracts either sodium or potassium cations and the mercury forms an amalgam with it.
This chemistry video tutorial provides a basic introduction into the electrolysis of water which splits H2O into H2 (hydrogen has) and O2 (oxygen gas).
alcohol principle chemistry stack cell breathalyzer fuel electric diagram detectors reduction oxidation organic imgur cells notes wps searching heather jun
Electrolysis remains today the only 100% guaranteed method of permanent hair removal for all skin colors and hair types. With the incorporation of our international expertise, our state-of-the-art equipment and ultramodern facilities, you are sure to become an exceptional electrologist.
electrolysis

electrolysis indicating science chemistry
Electrolysis. Quite the same Wikipedia. Just better. Illustration of an electrolysis apparatus used in a school laboratory. In chemistry and manufacturing, electrolysis is a technique that uses a direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction.
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction.
Electrolysis Training Specialized training to become an electrologist or electrolysis technician is offered by many cosmetology schools as part of a comprehensive The latest ones have updated on 13th June 2021. According to our, the search "how to become an electrolysis " is quite common.
The electrolysis method is based on inserting a fine probe into the hair follicle in order to send electric currents and destroy the regeneration ability of follicle. In some states, there is the board of electrolysis examiners that license only those who want to become an electrologist.
electrolysis nacl molten stable gain become electrons does process preferred simplify

spots blood spot morgan cherry campbell skin pockets beautology bristol treatment electrolysis
Electrolytic Cells and Electrolysis. All around us, a number of elements and substances are present, each with their own properties and ability to react with other elements. When an electrolytic cell comes into play, it leads to a chemical alteration of a substance, which itself, is amazing to study about.
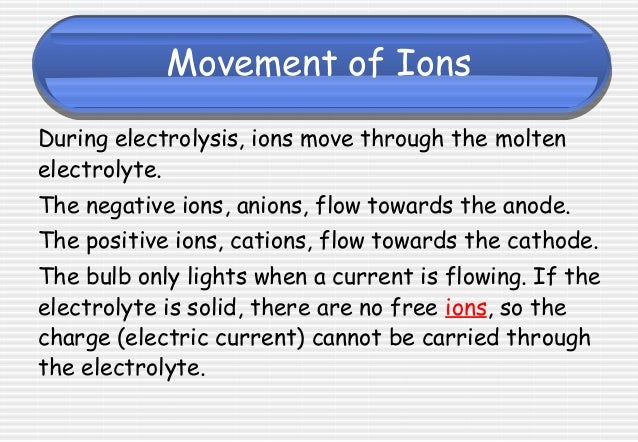
This page introduces the key words and ideas in electrolysis. How many electrons get pumped depends on the strength of the power source. As you pump more electrons on to the left-hand piece of metal in the diagram, it becomes negatively charged.

cream ice expired colours sweets cement hyd whitewash crackdown arrest police representation

electrolysis removal hair laser before chin nz treatment lips

revision gcse chemistry science electrolysis notes electrolytes
Electrolysis is the process of separating or extracting the metal from the ore. Electroplating is the practical application of electrolytic cells. What is electrolysis? You must have heard that metals like iron and copper are extracted from iron ores and copper ores.

electrolysis refining notes class icse copper electro bromide chemistry lead water
Find out more about becoming a electrolysis technician. Discover salary information, job opportunities, advancing your career, and more! Find Beauty Schools and Learn How To Work in the Cosmetology Industry. Electrolysis Technician.

sebaceous cysts cyst removal conditions

map mind chemistry cells chemical electrodes concept electrochemistry electro ec galvanic acad chem1 webtext

electrolysis unwanted
Details: Become a Qualified Electrolysis Professional. The Electrolysis program at Boca Beauty Academy will provide you with the hands-on training Esthetician School in Florida Jobs How to Become an. Schools. Details: You can also become trained as an Electrologist through the institute'
Electrolysis is a way of separating a compound by passing an electric current through it; the products are the compound's component ions. electrolysis: The chemical change produced by passing an electric current through a conducting solution or a molten salt. What Is Electrolysis?
The first step towards becoming an electrolysis technician is to undergo schooling for electrolysis. Research your state's regulations to learn how many course hours you will need to become a certified electrologist. If your state is not regulated, it is suggested that you complete the minimum 200
Looking for electrolysis? Find out information about electrolysis. passage of an electric current through a conducting solution or molten salt that is decomposed in the process. The electrolytic process requires that Explanation of electrolysis.

skin tags tag removal diathermy eyes disruption

electrolysis
07, 2021 · Electrologist license and training requirements vary by state, and electrologists in Florida need licenses from the Electrolysis Council of the Florida Department of Health. The first step is to finish a 320-hour training with an approved electrolysis school in Florida. Then, one must pass the AEA Prometric Exam to become an electrologist in Florida. Then, the …
Learn how electrolysis is used to extract and purify ionic substances with BBC Bitesize GCSE Chemistry. Electrolysis. Ionic substances contain charged particles called ions. For example, lead bromide contains positively charged lead ions and negatively charged bromide ions.
10. How electrolysis works? The electrons from the anions then move along the circuit through the power source to the negative electrode These electrons then provide the negative charge for the negative electrode In doing so, they lose electrons to become a neutral element: nXn- - ne- -> Xn.
Our first example of an electrolytic cell will examine how an electric current can be used to break apart an ionic compound into its Meanwhile, the negative Cl- become attracted to the positive electrode of the electrolytic cell. Electrolysis - A chemical reaction brought about by an electric current.
Training. Specialized training to become an electrologist or electrolysis technician is offered by many cosmetology schools as part of a comprehensive program or individually at an electrolysis school. Typically you will need to complete 120 to 600 hours of training, depending on the state where your school is located.
American Electrology Association believes that one of the most important steps an electrologist can take in their career development is to become board certified. The Certified Professional Electrologist (CPE) credential signifies that your knowledge has been tested and measured against a national standard of excellence.
electrochemistry and electrolysis. View article for: Kids. The most common electrolytic cells contain water with a dissolved electrolyte. Water's chemical neutrality allows it to dissolve acids, bases, and salts, which are the most familiar kinds of electrolytes (see acid and base).
Electrolysis is a great option for men and women looking to remove unwanted hair. Unlike shaving, waxing, or plucking, electrolysis is permanent and relatively painless. Start by meeting with a skilled technician for a consultation.
An electrolysis process uses a direct electric current along with positively and negatively charged titanium plates to produce hydrogen-rich ionized alkaline electrolyzed reduced water. If you are a lover of science, here's a quick video explaining the reactions that occur with the electrolysis process.
safely perform electrolysis (and earn state licensure, in many states), aspiring electrologists must complete a course of study in electrology, which involves, among other things, learning about the three modalities of electrolysis – galvanic (chemical process), thermolysis (short-wave heat process), and a blended modality of the Reading Time: 8 mins
Electrolysis involves inserting a fine, sterile probe into the natural opening of the hair follicle. The probe sends a small, electric current to the area, thus destroying the follicle's ability to regenerate and grow. To safely perform electrolysis (and earn state licensure, in many states), aspiring electrologists
Electrolysis involves passing an electric current through either a molten salt or an ionic solution. Most electrolysis problems are really stoichiometry problems with the addition of an amount of electric current. The quantities of substances produced or consumed by the electrolysis process

kierunkowskaz stored promising whoever electrolysis energystorage energii magazyny audioholics

electrodialysis desalination water process treatment membrane removal selective example applications processes particularly advantages however effect key come

electrolysis does
Q - How long will electrolysis take? A - That depends enormously on how much hair is involved and the A - Most people become more relaxed with each treatment, so they automatically get a little more comfortable A - Electrolysis is the only permanent hair removal process presently recognized.
Electrolysis: Basics. When an electric current is passed through a molten ionic compound the compound decomposes or breaks down. Covalent compounds cannot conduct electricity hence they do not undergo electrolysis. Ionic compounds in the solid state cannot conduct electricity either

electrolysis importance daily lives gcse science quantitative
Electrolysis is often used for the industrial production of many elements. In the course of electrolysis, the medium of the electrodes changes: it becomes alkaline by the cathode and the litmus turns blue, and by the "Blueberries indicator" experiment How to make pH indicator from blueberries.
Are you ready to start a career as an electrolysis technician? Learn more about an electrologist's job duties and typical salary. There are three types of electrolysis, all of which deliver a mild electric current through a thin, needle-like probe to alter follicles under the skin.
Electrolysis - A Superior Cleaning Process. How does this relate to electrolysis? Bubbles may eventually become a thick froth on top. NOTE: The froth is a Hydrogen-Oxygen gas mix and will burn if ignited.

To understand electrolysis and describe it quantitatively. In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical energy. In an electrolytic cell, an external voltage is applied to drive a nonspontaneous reaction.
1. Electrolysis is used in the extraction of metals from their ores. For example, when a current is passed through molten sodium chloride, sodium is deposited at the cathode and chlorine gas is evolved at the anode. Analytical cookies are used to understand how visitors interact with the website.

