Xolair (omalizumab) for provider administration is proven for patients with moderate to severe persistent asthma who meet all of the following criteria: Have a positive skin test or in vitro reactivity to a perennial aeroallergen; and Symptoms inadequately controlled with inhaled corticosteroids; and
How to Apply for a PRS Account. How to Register Your Study. A Phase IIIb Multicenter, Randomized, Double-Blind, Placebo-Controlled Study of Xolair in Subjects With The subcutaneous dose of Xolair administered in this study was either a minimum of mg/kg/IgE (IU/mL) every
To determine how much XOLAIR to administer in a dose, and how frequently to administer XOLAIR in adult patients 18 years of age or older, enter Health care providers administering XOLAIR should be prepared to manage anaphylaxis which can be life-threatening. Inform patients of the signs
are no guidelines specifying the care required following an omalizumab injections. The package insert does have a boxed warning for anaphylaxis and the statement in the package insert is “Administer only in a healthcare setting prepared to treat anaphylaxis that can be life-threatening and observe patients for an appropriate period of time” (Omalizumab Prescribing …
XOLAIR (omalizumab injection, solution) comes in different strengths and amounts. The appearance of XOLAIR can differ based on the dosing. Your doctor may change the dosage and prescription of XOLAIR to get you the best results possible.
Administration Overview. Administer XOLAIR by subcutaneous injection. XOLAIR is intended for use under the guidance of a healthcare provider. Can I take Xolair during pregnancy? Is Xolair an immunosuppressant? How long before Xolair starts working?
xolair injection how to administer. Xolair treats asthma in both adults and children. Specifically it seems designed for those with positive skin or blood allergy tests to ... This asthma patient explains how her quality of life has radically improved since she started taking Xolair injections.
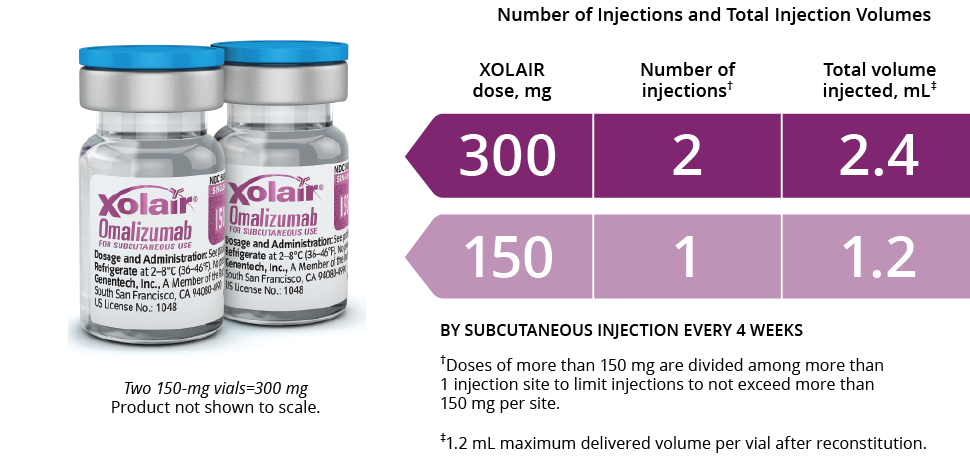
For subcutaneous administration only. Xolair must not be administered by the intravenous or intramuscular route. Doses of more than 150 mg should be divided across two or more injection sites. After proper training in subcutaneous injection technique, patients or the caregiver may
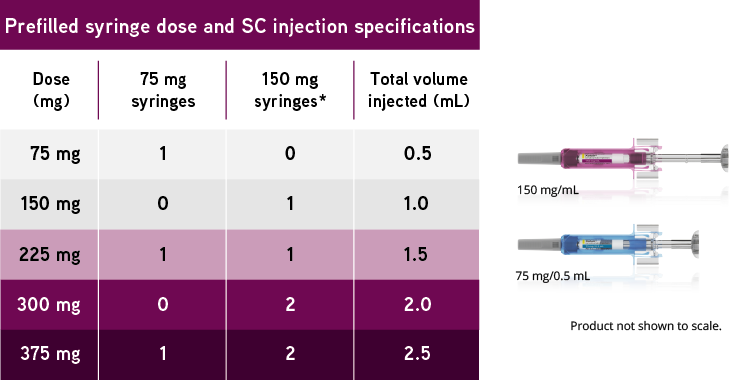
How Xolair Works. Xolair contains omalizumab, a monoclonal antibody produced by recombinant DNA technology. Xolair is administered subcutaneously every two or four weeks according to the dose determination chart below (Table 1). Doses (mg) and dosing frequency are determined by
15, 2018 · XOLAIR Self-Injection With Prefilled Syringe Is FDA Approved for Appropriate Patients as Determined by a Healthcare Provider. XOLAIR is intended for use under the guidance of a healthcare provider. Initiate therapy in a healthcare setting and once therapy has been safely established, the healthcare provider may determine whether self-administration of XOLAIR …
Xolair is indicated as add-on therapy to improve asthma control in patients with severe persistent allergic asthma who have a positive skin test or in Xolair treatment should be initiated by physicians experienced in the diagnosis and treatment of severe persistent asthma, chronic rhinosinusitis
"Appropriate patients will now have the flexibility to administer Xolair from home, which is particularly important for those who are considered high-risk The healthcare provider must train the patient or caregiver on the correct subcutaneous injection technique, how to recognize the signs and
Xolair (omalizumab) is a popular injectable anti-inflammatory drug used to treat asthma. "Anaphylaxis: Administer only and healthcare setting prepared to manage anaphylaxis that can be Contact us now to speak with one of our attorneys to discuss your legal options on how to
The Advocacy Council regularly receives queries from members on how to correctly code specific scenarios. Recently we received a request for assistance from a practice who received a denial for the administration of Xolair. Q: My claim for administering Xolair - using CPT 96372 - was denied.
What is Xolair and how is it used? Administer XOLAIR by subcutaneous injection. XOLAIR is intended for use under the guidance of a healthcare provider. Administer XOLAIR by subcutaneous injection. The injection may take 5-10 seconds to administer because the solution is slightly viscous.
Administer XOLAIR by subcutaneous injection. XOLAIR is intended for use under the guidance of a healthcare provider. Administer XOLAIR by subcutaneous injection. The injection may take 5-10 seconds to administer because the solution is slightly viscous.

pipe geckoes hative critters
XOLAIR Access Solutions can find out: • If your health insurance plan covers your medicine • How much your co-pay will be. Verify Information. Administer XOLAIR by subcutaneous injection. The injection may take 5-10 seconds to administer. Do not administer more than one injection per site.
Xolair® (omalizumab) prefilled syringe (PFS) is the first and only biologic to receive European Commission (EC) approval for self-administration in severe allergic asthma (SAA) and chronic spontaneous urticaria (CSU). Novartis is reimagining care in SAA and CSU by providing patients
XOLAIR® (OMALIZUMAB). Policy Number: PHARMACY T2. Effective Date: December 1, 2017. How Supplied. 150 mg vials 150 mg vials. National Drug Code. Omalizumab 300 mg administered every 4 weeks reduced weekly ISS and other symptom scores versus placebo
CONTRAINDICATIONS Xolair should not be administered to patients who have experienced a severe hypersensitivity reaction to Xolair (see WARNINGS: Anaphylaxis). Reconstituted Xolair vials should be protected from direct sunlight. HOW SUPPLIED Xolair (Omalizumab) is supplied as a
Administration Overview Administer XOLAIR by subcutaneous injection. XOLAIR is intended for use under the guidance of a healthcare provider. The injection may take 5-10 seconds to administer because the solution is slightly viscous. Do not administer more than 150 mg (contents of one vial)...
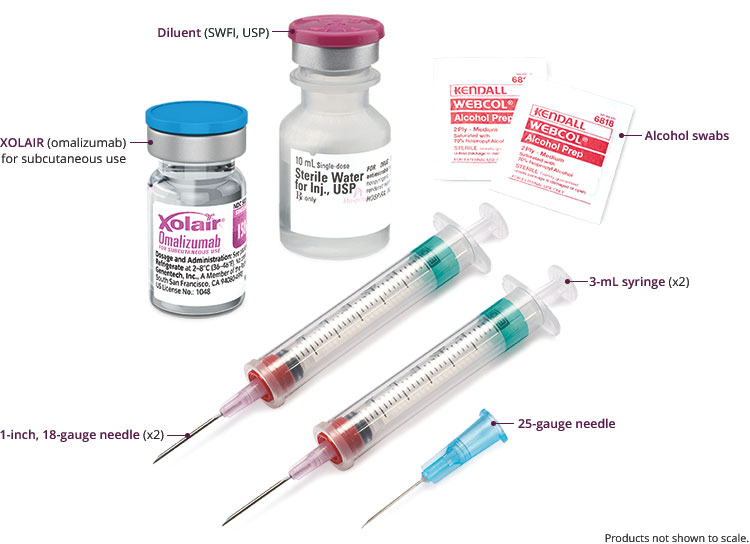
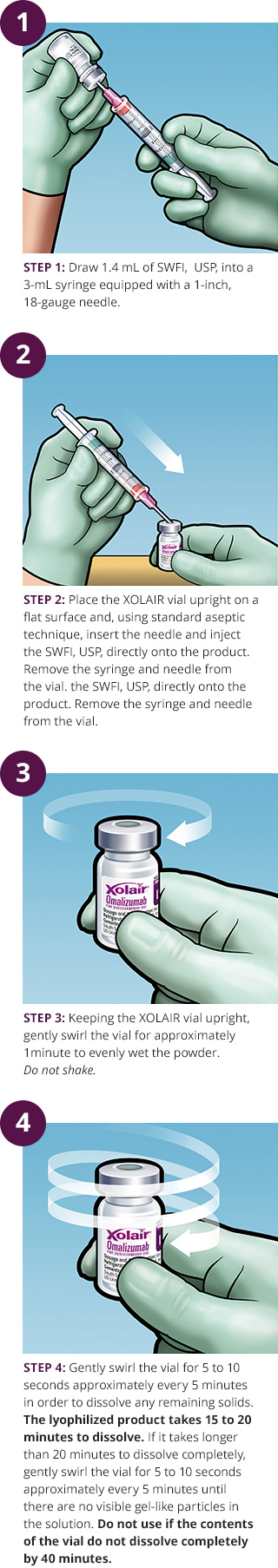
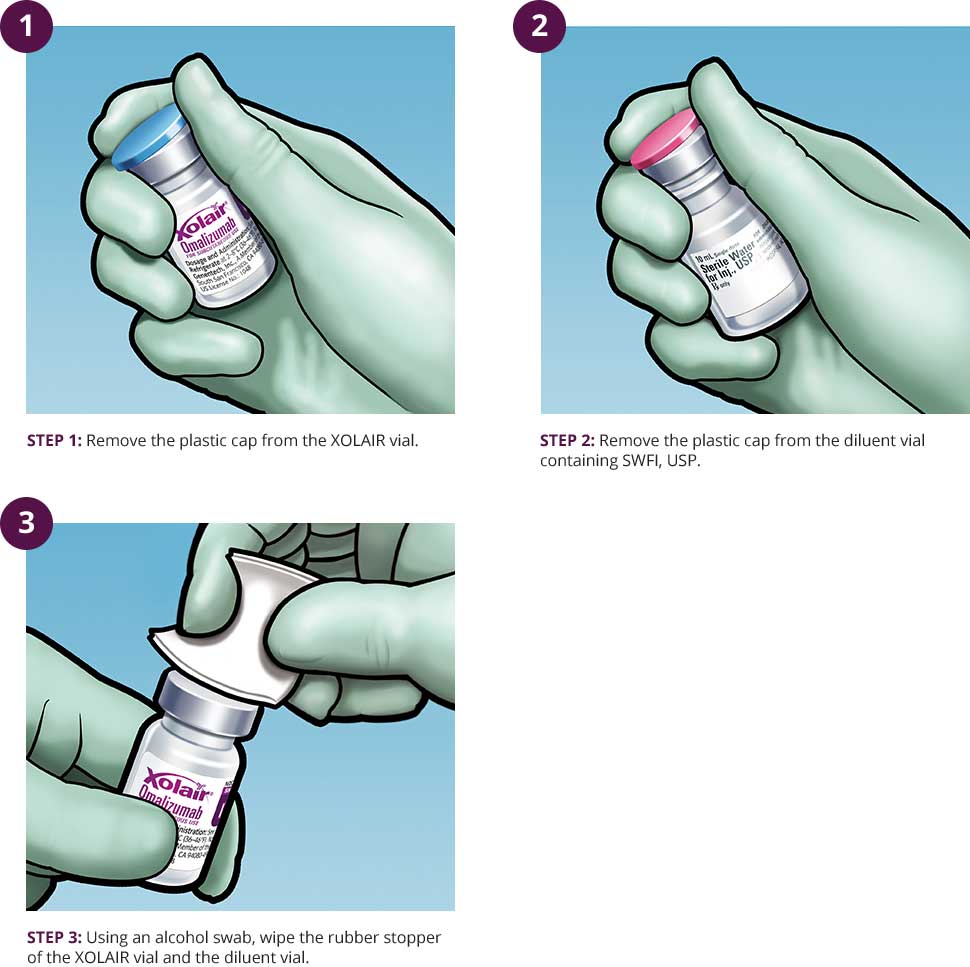
Find information on reconstituting and administering XOLAIR® (omalizumab) injections for subcutaneous use, including necessary supplies, step-by-step instructions and storage. See full safety & boxed warning.

Administration. XOLAIR is available as a prefilled syringe and as a lyophilized powder in vial for reconstitution. Both XOLAIR prefilled syringe and lyophilized powder should be administered by a Administer XOLAIR by subcutaneous injection. The injection may take 5-10 seconds to administer.
The XOLAIR Co-pay Program for drug or administration assistance may be used in a community practice, an alternate injection center (AIC), hospital outpatient department (HOPD) Ship XOLAIR to the treatment location. Doctor's offices, hopds and aics with a credit card terminal.
drawing human faces heads eyes features ears lips draw nose face mouth tutorial noses head learn mouths complete
Method of administration. For subcutaneous administration only. Xolair must not be administered by the intravenous or intramuscular route. There is limited experience with self-administration of Xolair powder and solvent for solution for injection. Therefore, treatment with this formulation
xolair dosing ciu omalizumab asthma injections allergic
you've established your XOLAIR treatment in a doctor's office or infusion center, your doctor will determine if you or a caregiver may inject XOLAIR. If your doctor decides that you or a caregiver may be able to give your own XOLAIR prefilled syringe injections, you should receive training on the right way to prepare and inject XOLAIR.
xolair reconstitution omalizumab reconstituting
xolair omalizumab
The FDA has approved a self-injectable formulation of Xolair for appropriate patients with moderate to severe persistent allergic asthma, nasal polyps or chronic If so, the provider must train the patient or caregiver how to administer the injection outside a health care setting, identify signs of
01, 2021 · Administer Xolair 75 mg to 600 mg by subcutaneous injection every 2 or 4 weeks based on serum total IgE level (IU/mL) measure before the start of treatment and by body weight (kg) [see Dosage and Administration ()]. Table 3. Subcutaneous Xolair Doses Every 2 or 4 Weeks* for Adult Patients with Nasal Polyps.
They must also be trained on how to self-administer Xolair. The approval covers countries in the EU, as well as Iceland, Norway and Liechtenstein. Novartis said Xolair's effectiveness has been demonstrated in large-scale clinical trials and real-world studies.
xolair dosing asthma omalizumab ciu prefilled injections allergic
They were administered Xolair in various dosages every 4 weeks. Forty-four percent of people receiving high-dose Xolair, and 22% of people receiving medium-dose Xolair The best data appears to be from a smaller study where the Xolair dose was similar to how it is given to a person with asthma.

xolair dosing ciu asthma omalizumab injections
Xolair is a popular injectable allergic asthma drug. Among its potential side effects are deadly heart attacks, mini-strokes and anaphylactic reactions. Xolair (omalizumab) is an injectable prescription medication used to treat moderate to severe persistent allergic asthma and chronic hives (a
Xolair. HMSA - Prior Authorization Request. CVS Caremark administers the prescription benefit plan for the patient identified. This patient's benefit plan 26. How long has the patient been diagnosed with CIU? Action Required: Please attach clinical notes supporting diagnosis of CIU for at least 3
XOLAIR® (omalizumab) should not be administered to patients with known hypersensitivity to omalizumab or any component of the formulation (see SUMMARY PRODUCT INFORMATION), or patients who have experienced a severe hypersensitivity reaction to XOLAIR®...
Can Xolair and immunotherapy be administered during the same clinic visit? Jean Murphree, MD. Omalizumab is safe to administer with allergy immunotherapy. Omalizumab binds to IgE and also decreases high-affinity receptors on mast cells, thus suppressing the inflammatory response.