The tight binding of biotin to streptavidin (Kd ' 10À13 M; Green, 1963) is the basis for its use in biotechnical applications where tight coupling of molecules is involved. The goal of our crystallographic studies was to determine how this biotin derivative binds to streptavidin.
Streptavidin /ˌstrɛpˈtævɪdɪn/ is a (tetramer) kDa protein purified from the bacterium Streptomyces avidinii. Streptavidin homo-tetramers have an extraordinarily high affinity for biotin (also known as vitamin B7 or vitamin H). With a dissociation constant (Kd) on the order of ≈10−14
using a biotin label, streptavidin conjugated to an enzyme such as horseradish peroxidase is used to detect the DNA fragment. [8] [9] While isotopic DNA labeling has little or no effect on protein binding affinity, use of non-isotopic labels including flurophores or biotin can alter the affinity and/or stoichiometry of the protein ...
capture antibody on a multi-well plate will immobilize the antigen of interest. This antigen will be recognized and bound by a detection antibody conjugated to biotin and streptavidin-HRP. An ELISA assay is typically performed in a multi-well plate (96- or 384-wells), which provides the solid surface to immobilize the antigen.
biotin is attached to a molecule, the molecule can be captured for detection, immobilization or affinity purification using conjugates or supports based on avidin or streptavidin proteins, which bind strongly and specifically to the biotin group.
Avidin, streptavidin and NeutrAvidin biotin-binding protein each bind four biotins per molecule with high affinity and selectivity. NANOGOLD gold clusters have several advantages over colloidal gold. They develop better with silver than do most gold colloids and therefore provide higher sensitivity.
Streptavidin is a tetrameric prokaryote protein that binds the co-enzyme biotin with an extremely high affinity. The streptavidin monomer is composed of In this tutorial, spheres within 6 angstroms of the ligand are used for the next step (with one sphere which does not belong to the active site
Streptavidin binds biotin conjugates with exceptional stability but dissociation does occur, limiting its Biotin-binding proteins have been isolated from a wide range of species, but streptavidin how derivatization at the carboxyl group changes binding strength. Traptavidin also had a
Since streptavidin does not bind specifically to many molecules other than biotin, and since streptavidin and biotin are highly resistant to dissociation, streptavidin is widely used to bind biotinylated proteins [68] . Using this biotin-streptavidin system, the protein transfer
, biotinylation (biotin-labeling) typically results in multiple biotin tags per antibody molecule, thus allowing more than one streptavidin molecule to bind to each antibody. Binding is aided by the fact that avidin-type proteins are tetrameric and have four biotin-binding sites per molecule.
This page focuses on the binding site for biotin and how two of the subunits collaborate in binding. What does the binding site look like? Let's display the. As you see, biotin is embedded inside streptavidin, but a considerable -and hydrophobic- part of the biotin molecule remains exposed.
photocleavable linker protein breaks uv

antibodies vector antibody labs
18, 2021 · Due to the high affinity of streptavidin-biotin interaction, harsh cell lysis and stringent washes that significantly reduce unspecific protein binding can be applied. We named this approach SUMO-ID.
Biotin binding reduces the tryptophan fluorescence emissions of streptavidin by 39%, blue shifts the emission peak from 333 to 329 nm, and reduces the bandwidth at half height from 53 to 46 nm. Acrylamide does bind to streptavidin (Ka = 5 M-1), and probably binds within the biotin-binding site.
Structural alterations at the biotin binding site produce quaternary changes in the streptavidin tetramer. These changes apparently propagate through cooperative deformations in the twisted beta sheets that link tetramer subunits.
Streptavidin Conjugates. A high quality biotin-binding protein conjugated to Biotium's signature bright and photostable fluorescent CF® dyes, and a selection of other This does not affect product stability or performance. If your coelenterazine is uniformly brown, then it is oxidized and needs to be replaced.
Streptavidin enhances cGAS binding to DNA and promotes cGAS phase separation. Whether and how bac-terial proteins bind cGAS to modulate innate immunity remain elusive. In addition, YRNA deletion did not signicantly affect streptavidin binding to biotin (-tagged histone peptides) in

foldit fold science protein games solve puzzles patriots england sepsis folding proteins cheerleader structure problem electrical unix play cure 2009

lsd1 epithelial histone gene
Streptavidin binds biotin in a similar man- ner to avidin, and it is reasonable to assume that avidin also binds 2-iminobiotin in a manner sim- ilar to streptavidin. A previous near-IR Raman study of the avidin- 2-iminobiotin complex reports small changes to the Trp contributions and a similar change
Streptavidin is a biotin-binding protein found in the culture broth of the bacterium Streptomyces avidinii. As a result, Streptavidin frequently exhibits much lower non-specific binding than avidin does.
The streptavidin-biotin system is a protein-ligand interaction present in nature that has been successfully used in a number of applications including Streptavidin, isolated from bacteria, binds to biotin equally well but lacks the glycoprotein portion found on avidin and therefore shows
(2) In the absence of biotin binding, the open-state streptavidin is more stable, which is consistent with experimental evidences. The free energy (ΔG) difference is about 5 kcal/mol between two states. But with biotin binding, the closed state is more stable due to electrostatic and
But did you know there are numerous forms of biotin that can be used for such applications? The affinity of the biotin-streptavidin interaction is extremely strong, with a disassociation constant (Kd) of 10−15 Biotin-TEG is commonly used to avoid hindrance issues and can be beneficial for
used with an appropriate substrate, the biotin-streptavidin system is very robust and can be optimized to achieve detection limits equal to those obtained with radioactive probes. It is important to note that performing such blotting assays with nucleotides requires the use of positively charged membranes in order to capture and immobilize ...
components of the avidin-biotin complex are commercially available in kits and the complex can be used with our biotinylated antibodies. The presence of endogenous biotin in tissues can bind the avidin-biotin complex and cause background staining. Such tissues include kidney, liver, brain, prostate, colon, intestines, and testes.
The binding of streptavidin to biotin is well known for the strong noncovalent interaction with femtomolar affinity (Kd = 10−14).1 The high affinity, slow exchange rate, and good specificity of the biotin-streptavidin interaction has resulted in a wide range of biotechnological applications

streptavidin enrichment sequencing labeling
This does not alter our adherence to all the PLOS ONE policies on sharing data and materials. Introduction. The extremely high affinity of biotin The avidin and streptavidin proteins are tetramers in solution. If the binding of the ligand is positively cooperative, differences in kon for initial and
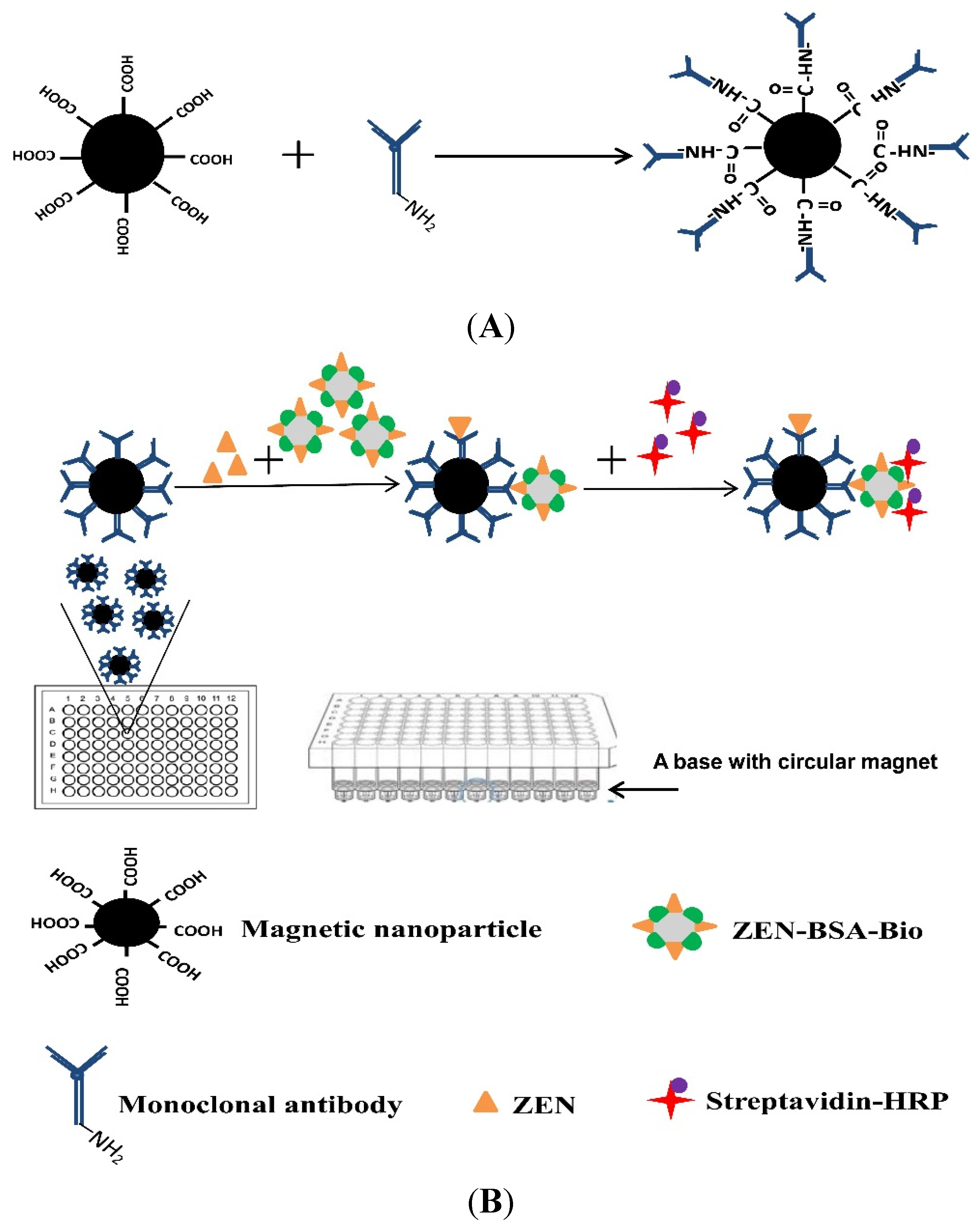
assay mnp nanoparticle immunosorbent toxins zearalenone nanoparticles immunomagnetic quantification
The high-anity binding of biotin to avidin, streptavidin, and related proteins has been exploited for decades. However, a disadvantage of the Streptavidin-based ligands can be gently stripped from desthiobiotin-labeled targets with buered biotin solutions. Thus, repeated probing with
When streptavidin was titrated with biotin, only two major bands were observed on the gel, consisting of streptavidin molecules without bound biotin and those @article{Sano1990CooperativeBB, title={Cooperative biotin binding by streptavidin. Electrophoretic behavior and subunit association
01, 2012 · When the detection antibody is labeled with biotin, you have the flexibility to use a number of different types of streptavidin conjugated reporters. Competitive Binding Assay A competitive binding assay is based upon the competition of labeled and unlabeled ligand for a limited number of antibody binding sites ( Figure 2 ).
The binding affinity of biotin to streptavidin and avidin is one of the highest reported for a non-covalent interaction to date, with a KD ∼10−14 M [4] 5. Conclusions. This work reports on how the reaction temperature influences the binding thermodynamics of the streptavidin-biotin interaction.
How can I effectively disrupt the biotin-streptavidin interaction so that I would be able to quantify the compound by measuring the florescence. can also try to add an excess of biotin so that the biotin competes with the biotin bound to streptavidin. all this does not work, it might worth
Streptavidin-Biotin Immunostaining of Paraffin-Embedded Tissue Sections. Do not allow slides to dry out after the fixation step, as drying will result in damage to the tissue structure. Beware, certain substrates are soluble in alcohol - please refer to supplier information for details.
Since streptavidin is multivalent (binding four molecules of biotin per tetrameric protein molecule) it may be used in combination with biotinylated antibody Unproteolyzed and proteolyzed preparations of streptavidin appear to bind biotin with equal affinity. The most highly proteolyzed tetramers
Although the biotin binding affinities of avidin and streptavidin are similar, the two proteins differ significantly in their amino acid compositions; however Importantly, he indicated that the free base of iminobiotin was the species that binds to avidin and that the iminobiotin-avidin dissociation
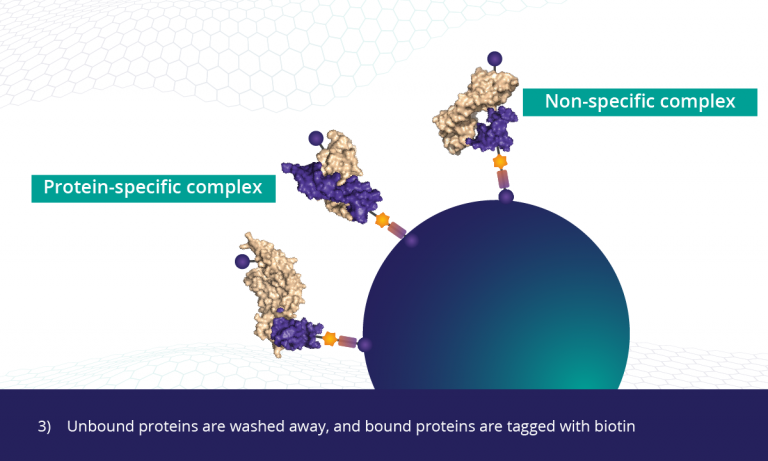
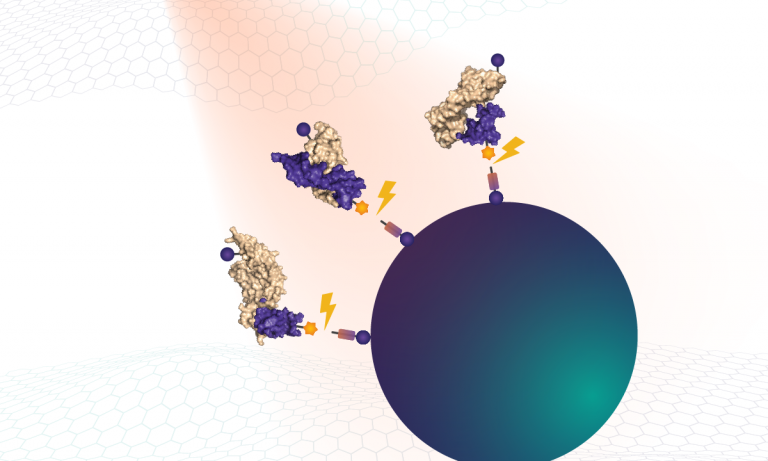
proteins somalogic unbound biotin
Avidin and other biotin-binding proteins, including Streptavidin and NeutrAvidin protein, have the ability to bind up to four biotin molecules, as shown in Although the Avidin-biotin system is simple to set up and use, it does have certain limitations. Because any biotinylated molecule will bind to

camp assay alphascreen synergy quantification microplate reader mode multi using competitive schematic figure biotek resources
Binding of streptavidin to the biotin groups results in an overall layer thickness of d = ( + ) nm that demonstrates the formation of. a well-ordered protein monolayer with the (biotin + spacer) units of the functionalized lipids being fully embedded into the binding pocket of the proteins.

biotin avidin fluorescent streptavidin complexes abcs
Thermophoretic behavior of a free protein changes upon ligand binding and gives access to information on the binding constants. In this work, we perform systematic thermophoretic measurements of the protein streptavidin (STV) and of the complex STV with biotin (B)
and biochemistry of the Strep-tag. Streptavidin is a tetrameric protein expressed in Streptomyces of its high affinity for vitamin H (), Streptavidin is commonly used in the fields of molecular biology and Strep-tag was originally selected from a genetic library to specifically bind to a proteolytically truncated "core" version of streptavidin.
plates are often used to create generic plates for solid-phase (coated plate) assays, such as ELISA assays, DELFIA® immunoassays, and FlashPlate® assays. Streptavidin will bind biotinylated antibodies, biotinylated proteins, and other biotinylated moieties, anchoring the biotinylated reagent to the well of the plate.
Biotin's affinity for streptavidin enables the biotinylated antigen/antibody complexes to bind to Once bound onto a streptavidin-coated solid phase, the interaction generates a signal that is What can institutions do to minimize the risk of biotin interference? When biotin interference is
The effect of biotin binding on streptavidin (STV) structure and stability was studied using Streptavidin-Biotin Interaction. TABLE I Thermodynamic parameters of thermal denaturation of the. Binding of biotin to STV does not change the -sheet structure content; that remains at 40%, but
